Immunotherapy: from predictive factors to antibiotics
Update of CheckMate 9LA
Based on the randomized, phase III CheckMate 9LA study, the first-line regimen of nivolumab plus ipilimumab and two cycles of chemotherapy has been approved in the indication of metastatic NSCLC without EGFR or ALK aberrations in many countries. CheckMate 9LA included approximately 360 patients with stage IV or recurrent disease in each arm and demonstrated significant OS, PFS, and ORR improvements with the immunotherapy-based regimen compared to four cycles of standard chemotherapy [1]. Reck et al. reported updated efficacy and safety findings after a minimum follow-up of 2 years, as well as outcomes in patients who discontinued treatment due to adverse events [2].
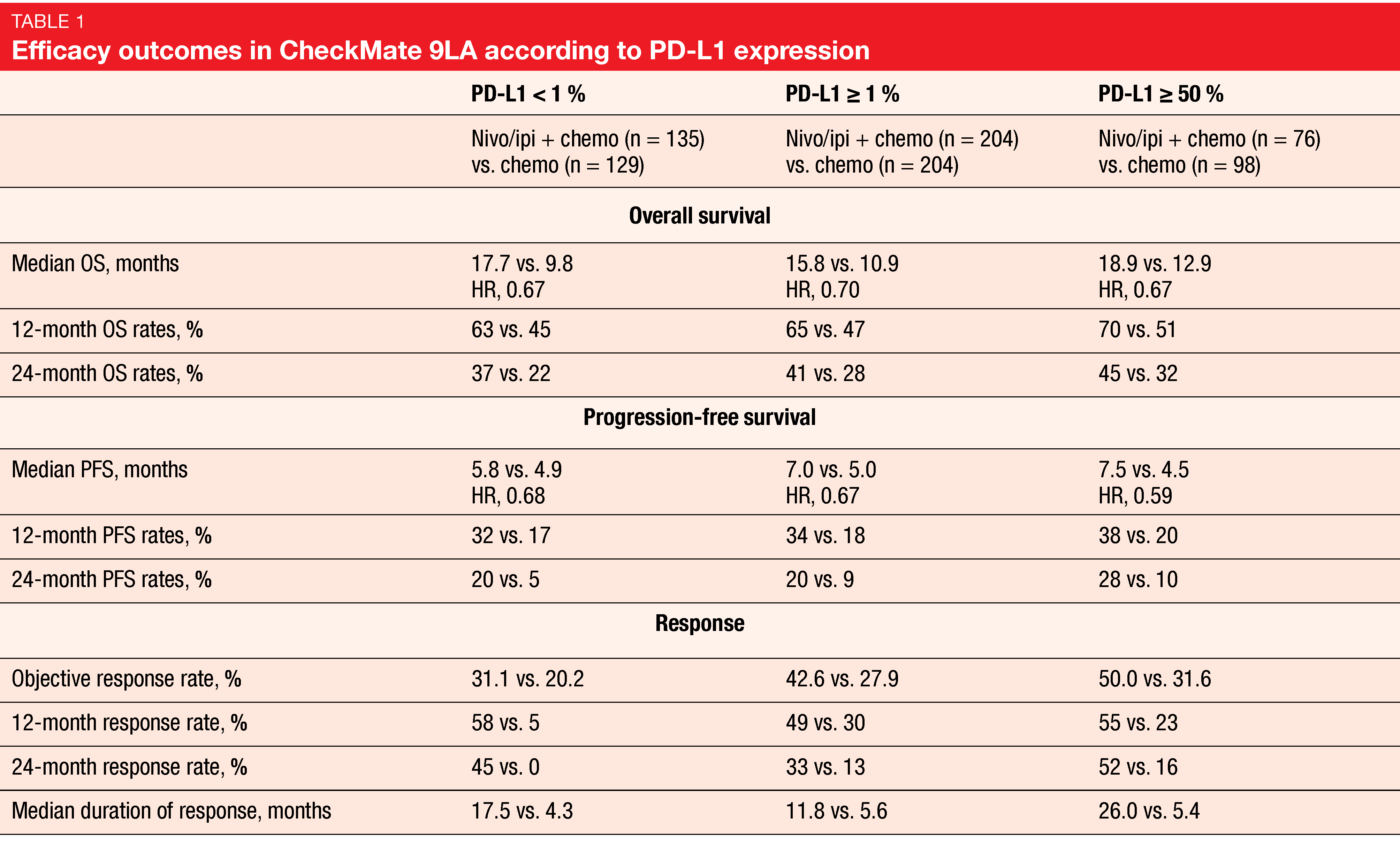
For overall survival, which constituted the primary endpoint, the analysis showed durable efficacy of the combination. Median OS was 15.8 vs. 11.0 months (HR, 0.72), with 24-month OS rates of 38 % vs. 26 %. Survival advantages occurred across all subgroups including patients with CNS metastases. Furthermore, PFS and response benefits were maintained with longer follow-up. At 24 months, 20 % vs. 8 % of patients in the experimental and control arms, respectively, were progression-free (HR, 0.67). Median duration of response was 13.0 vs. 5.6 months; here, the 24-month rates amounted to 34 % vs. 12 %. The combination proved superior to chemotherapy in all PD-L1 expression categories (i.e., < 1 %, ≥ 1 %, ≥ 50 %) in terms of OS, PFS, and response (Table 1). Similarly, patients treated in the experimental arm fared better with respect to OS in both non-squamous and squamous histology subgroups. No new safety signals were observed with longer follow-up. Most grade 3/4 treatment-related AEs (TRAEs) in the experimental arm emerged during the two chemotherapy cycles at the beginning of treatment.
A post-hoc exploratory analysis assessed the outcomes of patients who had discontinued all components of nivolumab/ipilimumab plus chemotherapy due to TRAEs. This showed that discontinuation did not have a negative impact on the long-term benefits. On the contrary, when compared indirectly to the total population randomized to combination treatment, these patients experienced improved survival with median OS of 27.5 months and a 24-month OS rate of 54 %. Fifty-one percent responded to therapy. After discontinuation, median duration of response was 14.5 months, and 56 % maintained their responses for ≥ 1 year. In their summary, the authors noted that these updated results continue to support nivolumab/ipilimumab plus two cycles of chemotherapy as an efficacious first-line treatment option for patients with advanced NSCLC.
Association between irAEs and OS
Immune-related adverse events (irAEs) have been reported in up to 80 % and 95 % of patients receiving checkpoint inhibitor monotherapy and combination therapy, respectively [3]. Increasing evidence suggests that the occurrence of irAEs with PD-(L)1 inhibitor therapy might be predictive of improved outcomes [4-7]. Based on this assumption, the post-hoc exploratory analysis presented by Socinski et al. evaluated the association between irAEs and OS in the IMpower130, IMpower132 and IMpower150 first-line trials [8]. IMpower130 and IMpower132 have assessed atezolizumab plus different chemotherapy regimens, while IMpower150 tested bevacizumab in addition to atezolizumab plus chemotherapy [9-11]. Pooling of the three trials yielded a total of 2,503 patients who had been treated with either atezolizumab-containing regimens (n = 1,577) or control therapies (n = 926). Each of these two groups was divided into patients with and without irAEs.
In the atezolizumab arm, 48 % of patients had any irAEs 11 % of which were grade 3-5. In the control arm, this applied to 32 % and 5 %, respectively. Patients who experienced irAEs demonstrated longer median OS than those without irAEs in both arms. For the atezolizumab arm, this was 25.7 vs. 13.0 months (HR, 0.69), and for the control arm, 20.2 vs. 12.8 months (HR, 0.82). At 1, 3, 6 and 12 months, atezolizumab-treated patients with irAEs showed the most favorable OS findings compared to the other groups. Also, ORR was highest in this cohort (61.1 %) compared to atezolizumab-treated patients without irAEs (37.2 %) and patients in the control arm with irAEs (42.2 %) and without irAEs (34.0 %).
OS was further evaluated by grade of irAEs in the atezolizumab arm. Here, patients with grade 1/2 irAEs experienced more favorable survival at 1, 3, 6 and 12 months than those with grade 3-5 irAEs or those without any irAEs. Patients with grade 3-5 irAEs had the shortest OS, potentially due to treatment disruption or discontinuation. The authors concluded that this analysis suggests an association between irAEs and efficacy in patients with NSCLC and further supports the use of atezolizumab combined with chemotherapy with or without bevacizumab in the first-line setting.
Dual PD-L1/CTLA-4 inhibition: KN046
The recombinant humanized PD-L1/CTLA-4 bispecific antibody KN046 provides dual CTLA-4 and PD-L1 inhibition and offers reduced treatment-associated toxicity due to limited peripheral distribution. An open-label, multi-center, phase II study was conducted to evaluate KN046 in combination with standard-of-care doublet chemotherapy based on the hypothesis that durable responses and OS benefits can be improved using this combined approach [12]. Patients with stage IV NSCLC who were naïve regarding systemic treatment received KN046 5 mg/kg i. v. every 3 weeks in addition to carboplatin plus either pemetrexed or paclitaxel dependent on histology. Overall, 87 patients participated in the trial, with 51 (56.8 %) and 36 (41.3 %) showing non-squamous and squamous histology, respectively. More than half of the tumors in the total group (55.4 %) expressed PD-L1 (≥ 1 %). ORR and disease control rate (DCR) were defined as the primary endpoint.
Indeed, the regimen demonstrated promising clinical benefit as first-line treatment in stage IV NSCLC, particularly in patients with PD-L1–positive tumors and squamous histology. In the total population, ORR and DCR were 50.6 % and 87.7 %, respectively. For the non-squamous group, this was 45.8 % and 89.6 %, respectively, and for the squamous group, 57.6 % and 84.8 %, respectively. Median PFS in all patients amounted to 5.9 months. Those with squamous histology and PD-L1 ≥ 1 % achieved the longest median PFS of 10.8 months. In the PD-L1–positive, all-histology group, this was 6.7 months. Median OS had not been reached yet, with a 15-month OS rate of 74.9 %.
Among grade ≥ 3 treatment-emergent AEs (TEAEs), diarrhea (5.7 %), alanine aminotransferase increases (4.6 %), infusion-related reactions (3.4 %) and rash (3.4 %) occurred most frequently. Grade ≥ 3 irAEs mostly comprised allergic dermatitis, diarrhea, and rash; overall, 8.0 % of patients developed at least one grade ≥ 3 irAE.
Cemiplimab in CNS disease
The phase III EMPOWER-Lung 1 study was performed to test the highly potent anti-PD-1 antibody cemiplimab as single-agent first-line treatment against chemotherapy according to investigator’s choice in patients with advanced NSCLC that showed ≥ 50 % PD-L1 expression. Compared to chemotherapy, the antibody treatment led to significant improvements with respect to OS, PFS, ORR, and duration of response [13]. EMPOWER-Lung 1 included a notable proportion of patients with brain lesions because the protocol permitted treated, clinically stable CNS metastases. Historically, these patients have been under-represented in clinical trials of first-line PD-(L)1 inhibitors [14-16]. A post-hoc subgroup analysis on the benefit of cemiplimab in patients with brain metastases was reported at ASCO 2021 [17].
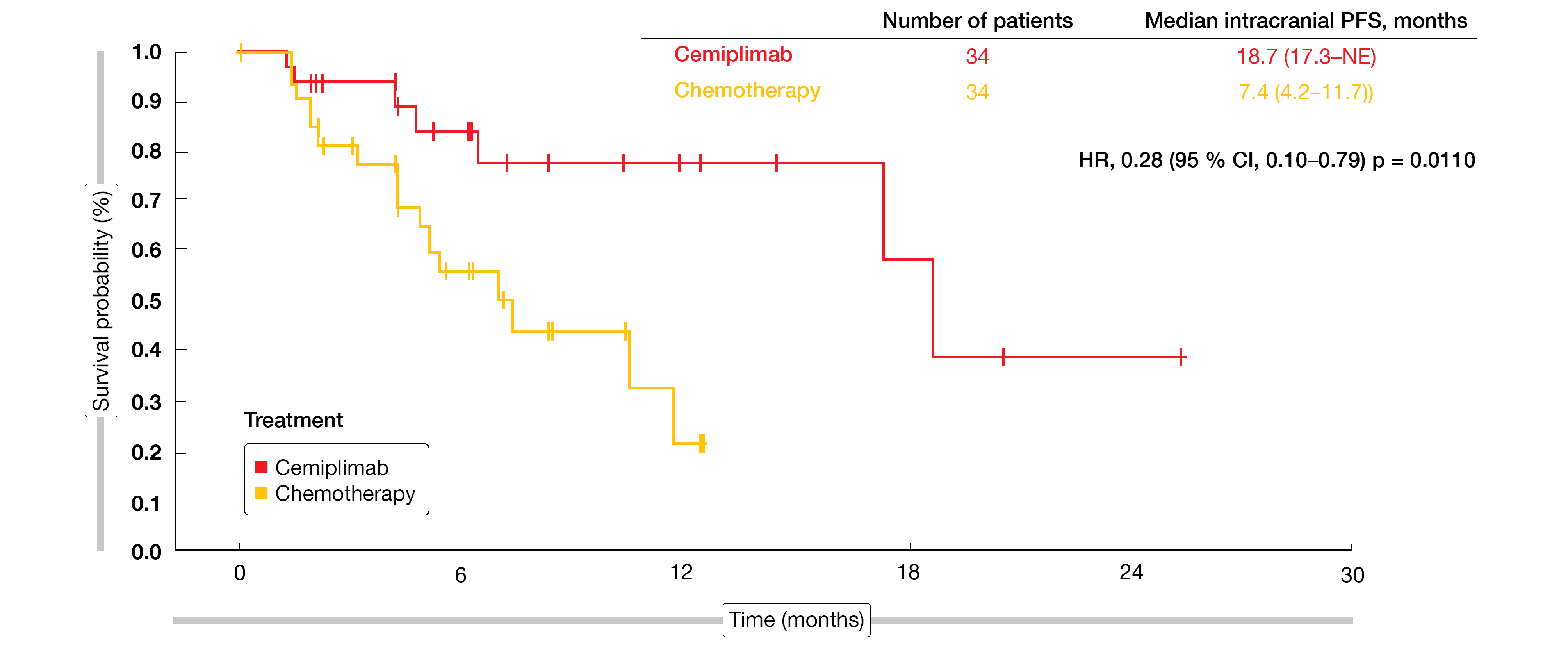
At the time of randomization, 68 of 563 patients (12.1 %) had treated stable brain metastases. They were evenly distributed between cemiplimab (n = 34) and chemotherapy (n = 34). In this group, cemiplimab gave rise to an OS advantage with an 83 % risk reduction (18.7 vs. 11.7 months; HR, 0.17; p = 0.0091). Median PFS was almost double, at 10.4 vs. 5.3 months (HR, 0.45; p = 0.0231). For intracranial PFS, the difference was even larger (18.7 vs. 7.4 months; HR, 0.28; p = 0.0110; Figure 1). Likewise, ORR by independent review committee in the cemiplimab group markedly exceeded the response rate obtained in the chemotherapy group (41.2 % vs. 8.8 %; OR, 6.9; p = 0.0034). Three patients in the experimental arm (8.8 %) achieved complete responses, while none in the control arm did. In their conclusions, the authors emphasized that the magnitude of clinical benefit observed with cemiplimab in patients with brain metastases compared favorably with the overall EMPOWER-Lung 1 population. Cemiplimab monotherapy was shown to represent a suitable option for this subgroup of patients.
Figure 1: Intracranial progression-free survival with cemiplimab vs. chemotherapy
Quality-of-life data for tislelizumab
The anti-PD-1 antibody tislelizumab has been engineered to minimize binding to Fcγ receptors on macrophages, thus abrogating antibody-dependent phagocytosis, which is a potential mechanism of resistance to anti-PD-1 therapies [18, 19]. RATIONALE 303, a randomized, open-label, multicenter, phase III trial, has demonstrated significant OS, PFS and ORR benefits for single-agent tislelizumab compared to docetaxel in patients with NSCLC experiencing progression during or after a platinum-containing regimen [20]. At ASCO 2021, Zhou et al. presented findings on health-related quality of life as assessed in RATIONALE 303 using the EORTC QLQ-C30 and QLQ-LC13 questionnaires [21]. Overall, 805 patients had been randomized. The analyzed population included 784 individuals; among these, 530 and 254 had been treated with tislelizumab and docetaxel, respectively.
Changes in the EORTC QLQ-C30 scores from baseline favored the immune checkpoint inhibition. The patients in the experimental arm experienced improvements regarding global health score/quality of life and fatigue in both cycles 4 and 6 compared with those treated in the control arm. While the physical function domain worsened in the docetaxel arm in cycles 4 and 6, this remained stable with tislelizumab. The difference between treatments became significant in cycle 6. Similarly, compared with docetaxel, the EORTC QLQ-LC13 index score (overall symptoms), cough, and peripheral neuropathy improved significantly in the tislelizumab arm in both cycles 4 and 6. By cycle 6, there was a trend towards significant improvement for dyspnea. No differences occurred with respect to pain measures and hemoptysis. Also, tislelizumab-treated patients experienced a lower risk of deterioration in overall symptoms as indicated by the QLQ-LC13 index score, dyspnea, cough, and peripheral neuropathy.
The symptom improvements were tested using two types of analysis, with the results showing similar patterns. As the authors emphasized, these findings are in line with the clinical and survival benefits observed for tislelizumab [18] as well as other findings on health-related quality of life in the context of PD-1 inhibition [22]. The data add to the favorable risk-benefit ratio of tislelizumab in patients with NSCLC who have progressed on a platinum-containing regimen.
Tislelizumab plus chemotherapy
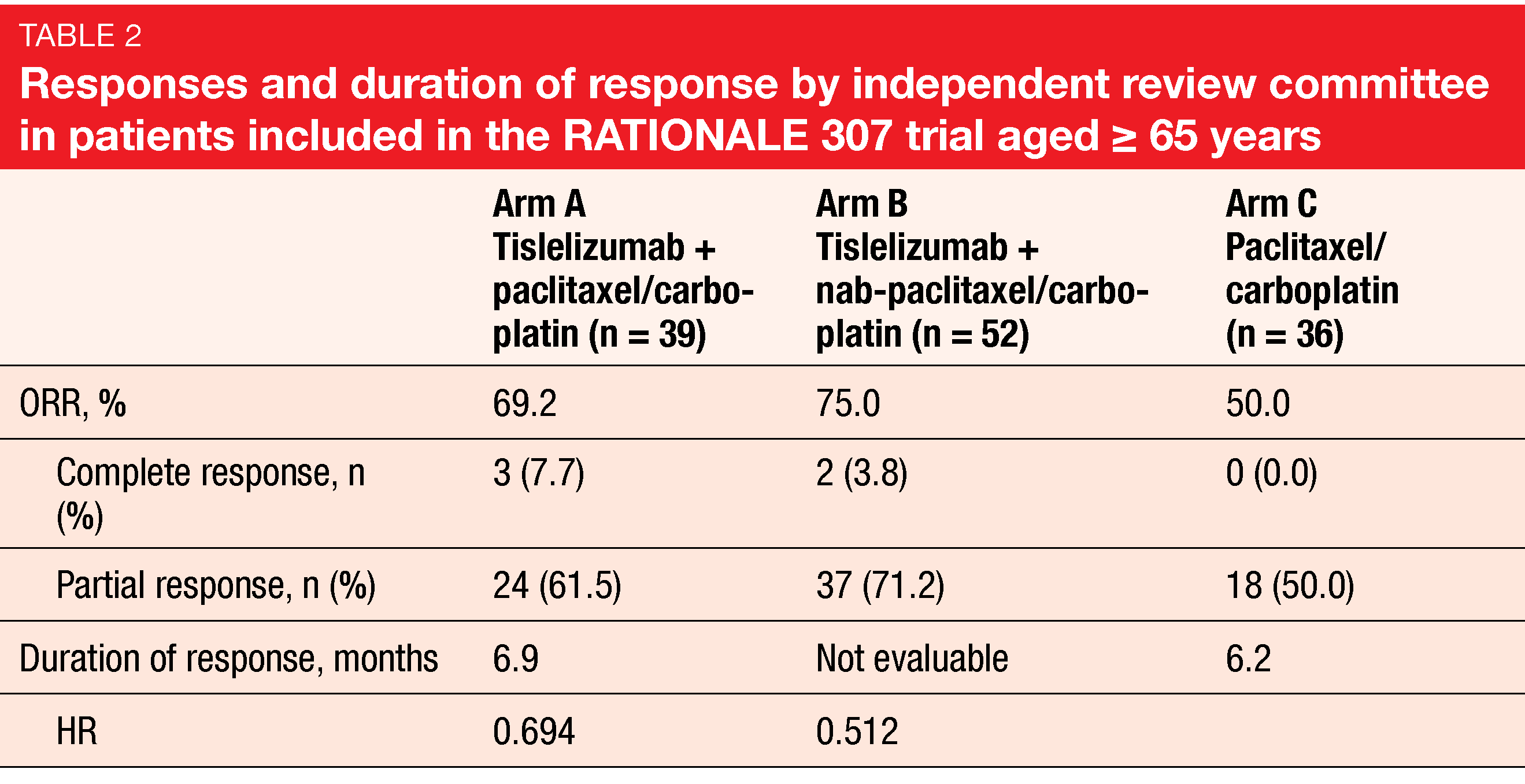
The open-label, randomized, multicenter phase III RATIONALE 307 trial assessed tislelizumab combined with chemotherapy as first-line treatment of patients with advanced NSCLC of squamous histology [23]. In addition to tislelizumab, Arms A and B received paclitaxel/carboplatin and nab-paclitaxel/carboplatin, respectively. Arm C was treated with paclitaxel/carboplatin. The combined approach significantly improved median PFS, with risk reductions of approximately 50 % for Arm A vs. C and Arm B vs. C (p < 0.001 each). Based on RATIONALE 307, tislelizumab has been approved in combination with chemotherapy for the first-line treatment of patients with advanced squamous NSCLC in China. Wang et al. reported a subgroup analysis of the trial conducted in patients aged ≥ 65 years [24]. Among 127 elderly study participants, 39, 52 and 36 had been randomized into Arms A, B, and C, respectively.
Tislelizumab plus chemotherapy elicited significant benefits in this group. PFS was longer in the tislelizumab-treated arms (9.7 months each) than in the chemotherapy-alone arm (5.2 months; HRs, 0.602 and 0.564). ORRs in Arms A and B exceeded the response rate obtained in Arm C (Table 2). The safety profile including irAEs was consistent with the profile observed in the overall population. TEAEs leading to permanent discontinuation of tislelizumab occurred with similar incidence in Arms A and B (15.4 % each). Confirmed immune-related TEAEs were reported in 35.9 % and 34.6 % in Arms A and B, respectively. Most were mild or moderate and did not lead to discontinuation of any treatment component.
Another tislelizumab-based combination is being assessed in the ongoing randomized, double-blind, phase III AdvanTIG-302 trial [25]. This global study will provide insights into the effect of dual targeting with tislelizumab and the anti-TIGIT antibody ociperlimab in patients with PD-L1-high, locally advanced/recurrent or untreated metastatic NSCLC. Anti-TIGIT and anti-PD1 antibodies have been shown to induce synergistic immune cell activation and enhanced antitumor activity [26]. AdvanTIG-302 is comparing ociperlimab plus tislelizumab (Arm A) with pembrolizumab (Arm B) and tislelizumab monotherapy (Arm C). PFS and OS for Arm A vs. Arm B have been defined as the dual primary endpoint.
Microbiome and IO activity
As antibiotic treatment disrupts the native gut microbiome that plays an important role in host response to immunotherapy, antibiotics are assumed to compromise the efficacy of immune checkpoint inhibitors in patients with NSCLC. Stokes et al. further explored this association in a large population from the Veterans Health Administration Database (n = 3,634) in their retrospective cohort study [27]. These patients had been diagnosed with NSCLC from 2010 to 2018 and treated with checkpoint inhibitors. Indeed, receipt of antibiotics within either 30 days before or 60 days after the start of immune checkpoint inhibition was associated with significantly lower survival (p < 0.0001). The authors stressed that antibiotics should be used judiciously in NSCLC patients receiving checkpoint inhibitors as they might exert a detrimental effect on outcomes.
Similarly, a prospective, multicentric, observational study related to the intestinal microbiome in the setting of immunotherapy [28]. It has been shown that the presence of the bacterium Akkermansia muciniphila correlates with the success of nivolumab treatment [29]. The present study aimed to validate the prognostic significance of Akkermansia in patients with advanced NSCLC amenable to immunotherapy in the first and second lines. Stool samples were collected from 311 patients at study entry and analyzed using metagenomics sequencing. Akkermansia was detected in 158 cases and absent in 153.
Relative abundance as a predictor
Objective response rate, which constituted the primary endpoint, was higher in the Akkermansia-positive group than in the negative population (27 % vs. 17 %). Likewise, most of the patients in the group of those who were alive at 12 months and beyond were Akkermansia-positive (57 %). In contrast, only 42 % of those who lived for < 12 months were positive. According to the authors, the presence of Akkermansia is indeed a surrogate biomarker of improved outcomes.
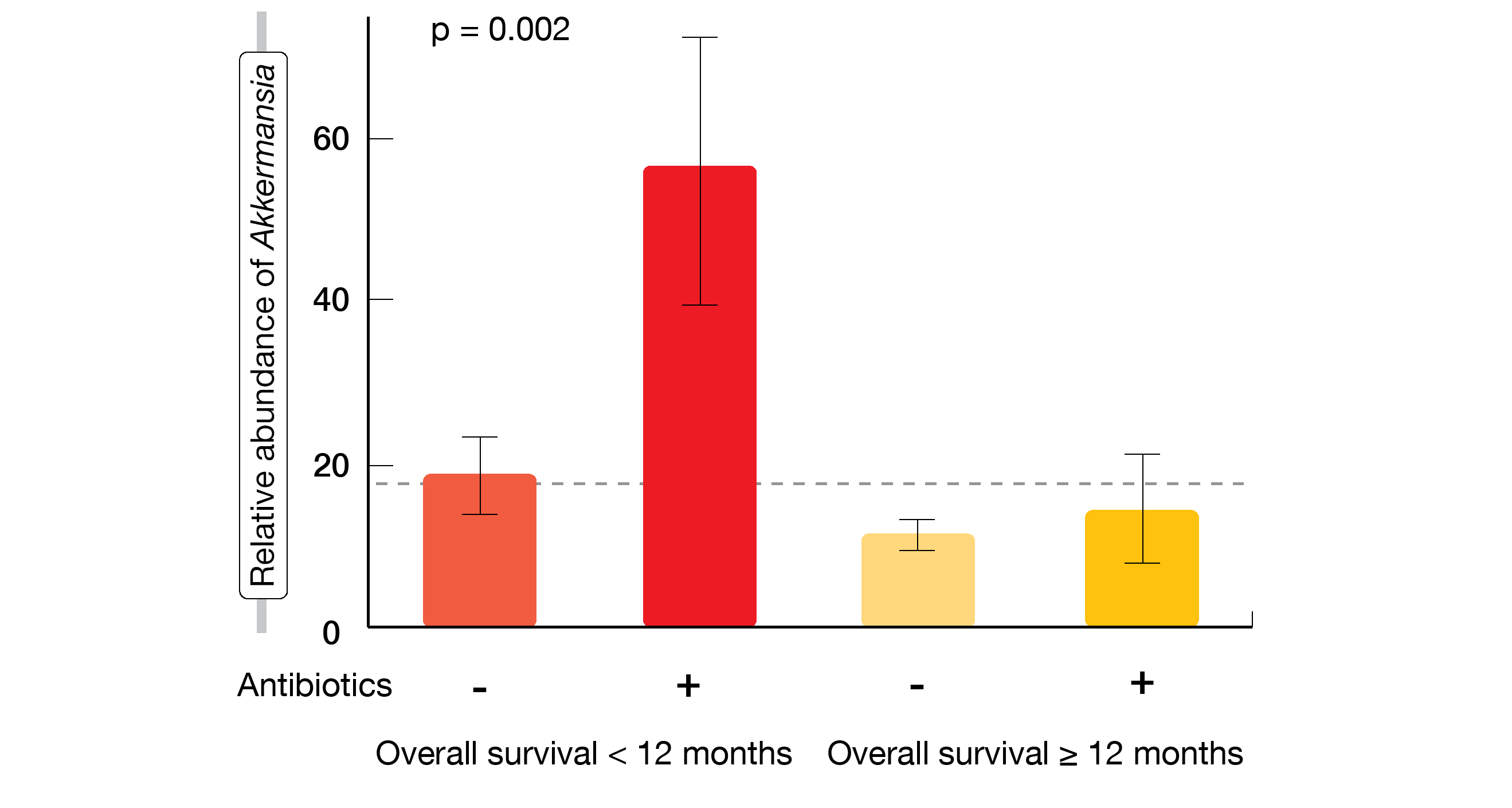
However, stratification based on the relative abundance of Akkermansia was shown to be a more accurate independent predictor than the binary modality. Akkermansia was unexpectedly over-represented in patients with OS < 12 months within the positive group. The researchers divided the cohort into three groups (negative, low, high) and showed that low relative abundance of Akkermansia correlated with increased ORR and OS, while patients with high relative abundance fared worst. Overabundance was more frequent in patients exposed to antibiotics than in those without antibiotic exposure. Patients who had both high relative abundance of Akkermansia and exposure to antibiotics were most likely to have short survival (Figure 2). RNA sequencing of tumor samples at diagnosis revealed increased expression of CD3, VCAM1 and ZBP1, which indicates that Akkermansia promotes recirculation of cells in the microenvironment.
The authors concluded that these data provide a rationale to develop a microbiome-based approach to study gut dysbiosis in routine clinical oncology care. The first immunotherapy trial for patients with advanced NSCLC and undetectable intestinal Akkermansia muciniphila will be launched by the study group.
Figure 2: Survival outcomes according to the presence of Akkermansia muciniphila and use of antibiotics
REFERENCES
- Paz-Ares L et al., First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22(2): 198-211
- Reck M et al., First-line nivolumab + ipilimumab + 2 cycles of chemotherapy versus chemotherapy alone (4 cycles) in patients with advanced non-small cell lung cancer: 2-year update from CheckMate 9LA. J Clin Oncol 39, 2021 (suppl 15; abstr 9000)
- Jamal S et al., Immune-related adverse events associated with cancer immunotherapy: a review for the practicing rheumatologist. J Rheumatol 2020; 47(2): 166-175
- Remon J et al., Immune-related adverse events and outcomes in patients with advanced non-small cell lung cancer: a predictive marker of efficacy? Thorac Oncol 2019; 14(6): 963-967
- Zhou X et al., Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020; 18(1): 87
- von Pawel J et al., Association between immune-related adverse events and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol 2017; 28(Supplement 5): v469
- Haratani K et al., Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4(3): 374-378
- Socinski MA et al., Pooled analyses of immune-related adverse events and efficacy from the phase 3 trials IMpower130, IMpower132 and IMpower150. J Clin Oncol 39, 2021 (suppl 15; abstr 9002)
- West H et al., Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(7): 924-937
- Nishio M et al., Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 Trial. J Thorac Oncol 2021; 16(4): 653-664
- Socinski MA et al., Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378(24): 2288-2301
- Yang Y et al., A phase 2, open-label, multi-center study to evaluate the efficacy, safety, and tolerability of KN046 in combination with chemotherapy in subjects with advanced non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 9060)
- Sezer A et al., Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021; 397(10274): 592-604
- Goldberg SB et al., Pembrolizumab for the management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020; 21(5): 655-663
- Goldberg SB et al., Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17(7): 976-983
- Abrey LE, Inclusion of patients with brain metastases in clinical trials. Clin Invest 2011; 1(8): 1065-1068
- Özgüroglu M et al., Cemiplimab monotherapy as first-line treatment of patients with brain metastases from advanced non-small cell lung cancer with programmed cell death-ligand 1 ≥ 50 %; EMPOWER-Lung 1 subgroup analysis. J Clin Oncol 39, 2021 (suppl 15; abstr 9085)
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090
- Qin S et al., RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol 2019; 15(16): 1811-1822
- Zhou C et al., Results from RATIONALE 303: a global phase 3 study of tislelizumab vs docetaxel as second-line or third-line therapy for patients with locally advanced or metastatic NSCLC. AACR 2021, abstract CT039
- Zhou C et al., The effects of tislelizumab treatment on the health-related quality of life non-small cell lung cancer patients who progressed on a prior platinum-containing regimen. J Clin Oncol 39, 2021 (suppl 15; abstr 9069)
- Brahmer JR et al., Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol 2017; 18(12): 1600-1609
- Wang J et al., Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7(5): 709-717
- Wang J et al., RATIONALE-307: tislelizumab plus chemotherapy versus chemotherapy alone as first-line treatment for advanced squamous NSCLC in patients aged ≥ 65. J Clin Oncol 39, 2021 (suppl 15; abstr 9102)
- Socinski MA et al., AdvanTIG-302: Anti-TIGIT monoclonal antibody ociperlimab plus tislelizumab vs pembrolizumab in programmed death ligand 1-selected, previously untreated, locally advanced, unresectable or metastatic non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr TPS9128)
- Rodriguez-Abreu D et al., Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab plus atezolizumab versus placebo plus atezo as first-line treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol 38: 2020 (suppl; abstr 9503)
- Stokes WA et al., Effect of antibiotic therapy on immunotherapy outcomes for non-small cell lung cancer: analysis form the Veterans Health Administration Database. J Clin Oncol 39, 2021 (suppl 15; abstr 9017)
- Derosa L et al., Intestinal Akkermansia muciniphila predicts overall survival in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies: results of a phase II study. J Clin Oncol 39, 2021 (suppl 15; abstr 9019)
- Routy B et al., Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359(6371): 91-97
© 2021 Springer-Verlag GmbH, Impressum
More posts
Opening up new vistas for patients with SCLC
Opening up new vistas for patients with SCLC Cisplatin vs. carboplatin in LS-SCL
Enhancing immunosupportive mechanisms via anti-angiogenesis
Enhancing immunosupportive mechanisms via anti-angiogenesis Treatment with anti-a
How does checkpoint inhibition perform in the setting of oncogene-driven lung cancer?
How does checkpoint inhibition perform in the setting of oncogene-driven lung cancer
Immunotherapy: from predictive factors to antibiotics
Immunotherapy: from predictive factors to antibiotics Update of CheckMate 9LA Bas
KRAS, MET, ROS1, HER2: current perspectives
KRAS, MET, ROS1, HER2: current perspectives CodeBreaK100: sotorasib Approximately
EGFR-mutant disease: strategies against sensitizing and resistance-mediating mutations
EGFR-mutant disease: strategies against sensitizing and resistance-mediating mutati