Immunotherapy: anti-tumour activity despite extensive pretreatment
The anti-PD-1-antibodies pembrolizumab and nivolumab have been shown to be active in lung cancer. Pembrolizumab is a high-affinity, humanised, monoclonal IgG4κ antibody against PD-1 that prevents the interaction of the receptor with PD-L1 and PD-L2.
The KEYNOTE-001 trial demonstrated significant anti-tumour activity of pembrolizumab in advanced NSCLC, with improved outcomes in terms of PD-L1 Tumor Proportion Scores (TPS) ≥ 50 % [1]. The TPS reflects the expression of PD-L1 on the tumour. The ≥ 50 % cut-off was determined using independent training and validation datasets from KEYNOTE-001.
Pembrolizumab is approved in the US for treatment of patients with advanced, PD-L1–positive NSCLC that has progressed after platinum-containing chemotherapy and appropriate TKI therapy for EGFR or ALK genomic aberrations.
KEYNOTE-010
Additional data are provided by the randomised phase II/III KEYNOTE-010 trial, which included patients with PD-L1-positive advanced NSCLC and disease progression after at least one line of chemotherapy [2]. Two doses of pembrolizumab (2 mg/kg every 3 weeks, or 10 mg/kg every 3 weeks, for 24 months) were compared with docetaxel (75 mg/m2 every 3 weeks, per local guidelines). Patients had to be PD-L1 positive. A TPS of ≥ 1 % was one of the inclusion criteria, and PD-L1 status was a stratification factor (i.e., TPS ≥ 50 % vs. 1 %–49 %). The following endpoints were assessed separately for the TPS ≥ 50 % and TPS ≥ 1 % groups: PFS and OS (co-primary), objective response rate (ORR), duration of response, and safety (secondary).
The screening included 2,699 patients, 1,475 of whom were PD-L1 positive with TPS ≥ 1 %. Initially, both archival biopsies and new tissue samples were allowed for the tumour analysis, although after an amendment, new tumour samples had to be used unless the risk of a biopsy was considered too high. Overall, 456 out of 1,034 randomised patients participated in the trial based on archival samples. Approximately 20 % in each arm had squamous-cell carcinoma. EGFR mutations were found in 8 %, 9 % and 8 % of patients treated with pembrolizumab 2 mg/kg, 10 mg/kg, and docetaxel, respectively. The analysis revealed a PD-L1 TPS of ≥ 50 % and 1 %–49 % in approximately 40 % and 60 % of the patients, respectively, across the treatment arms. Twenty percent to 30 % of the patients received the study treatment as third line or later lines.
Outcome improvements in patients with TPS ≥ 1 %
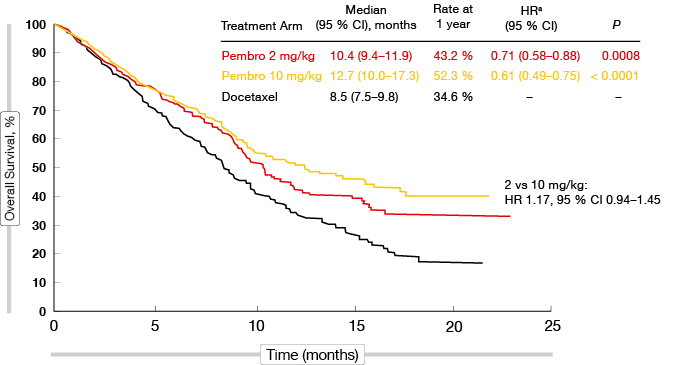
In the TPS ≥ 50 % group, pembrolizumab treatment at both doses gave rise to highly significant OS benefits compared to docetaxel chemotherapy (HR, 0.54, 0.50 with 2 mg/kg, 10 mg/kg, respectively; p = 0.0002, p < 0.0001, respectively). The median survival rates obtained with pembrolizumab were nearly doubled compared to docetaxel. The results for the TPS ≥ 1 % group were of particular interest, as these represented the majority of the patients. Again, the two pembrolizumab doses showed similar activities (Figure), with the mortality risk reduced by 29 % and 39 %, respectively, compared to docetaxel (p = 0.0008, p < 0.0001, respectively). The OS benefit emerged early on. All of the subgroups (i.e., sex, age, ECOG performance status, tumour sampling [archival vs. new], histology, EGFR status) benefited from the pembrolizumab treatment. This advantage of pembrolizumab also applied to both of the TPS groups (≥ 50 % vs. 1 %–49 %), which is relevant for treatment decisions.
For PFS, the analysis also favoured pembrolizumab treatment to a significant extent in both of the TPS groups. For the TPS ≥ 50 % group, the HRs were 0.59 for both pembrolizumab doses compared to docetaxel (p = 0.0001, p < 0.0001, respectively). For the TPS ≥ 1 % group, the HRs were 0.88 and 0.79, respectively (p = 0.07, p = 0.004, respectively). The response rates were highly significantly improved in the TPS ≥ 50 % group (30 %, 29 % vs. 8 %; p < 0.0001 for both comparisons), as well as in the TPS ≥ 1 % group (18 % for both pembrolizumab doses vs. 9 % for docetaxel; p = 0.0005, p = 0.0002, respectively). Also, the duration of these responses was considerably longer with both pembrolizumab doses than with docetaxel, irrespective of TPS.
Figure: Overall survival with pembrolizumab at two doses vs. docetaxel in the population with TPS ≥1 % (KEYNOTE-010)
Pembrolizumab: a new standard of care
Pembrolizumab treatment was well tolerated, with markedly lower rates of high-grade toxicity compared to docetaxel. Approximately half as many patients in the pembrolizumab arms versus the docetaxel arm discontinued treatment due to AEs. Immune-related events occurred with a maximum incidence of 8 %. The main immune-mediated AEs were hypothyroidism and hyperthyroidism, and most of these were rated as low grade. From 2 % to 3 % of the patients treated with the two pembrolizumab doses developed pneumonitis, and although this AE also emerged in the docetaxel-treated arm, it did so less frequently. Again, pneumonitis was low-grade in the majority of cases.
Overall, the data obtained from the KEYNOTE-010 trial validate the use of the PD-L1-positivity selection in advanced NSCLC. They support the treatment schedule of pembrolizumab 2 mg/kg every 3 weeks that is currently approved in the US for patients with advanced NSCLC. Moreover, these findings support pembrolizumab as one new standard of care for patients with advanced NSCLC that show disease progression on platinum-based chemotherapy.
Nivolumab in non-squamous NSCLC: CheckMate 057
The fully human IgG4 PD-1 immune checkpoint inhibitory antibody nivolumab has been approved in the US for treatment of patients with metastatic NSCLC whose disease has progressed on or after platinum-based doublet chemotherapy and after anti-EGFR or anti-ALK TKI therapies. In Europe, the approval extends to patients with locally advanced or metastatic squamous-cell NSCLC whose disease has progressed on or after prior chemotherapy.
The randomised phase III CheckMate 057 trial investigated nivolumab versus docetaxel in pre-treated patients with advanced non–squamous-cell NSCLC (independent of PD-L1 status). Docetaxel served as the comparator because it is a standard second-line treatment in this setting. This treatment can be expected to give rise to response rates of 9.0 % to 14.5 %, and median OS of 8.0 months to 10.4 months.
In CheckMate 057, the patients were randomised to either nivolumab 3 mg/kg every 2 weeks or docetaxel 75 mg/m2 every 3 weeks. Prior maintenance therapy was allowed, as well as prior TKI treatment for patients with known EGFR mutation or ALK translocation. The primary endpoint was OS, while the secondary endpoints included ORR, PFS, safety, efficacy according to PD-L1 expression, and patient-reported outcomes. Tumour tissue was available for PD-L1 expression analysis in approximately 80 % of the patients, and about half of these showed as PD-L1 positive. At the ESMO Asia Congress, the 18-month analysis of the CheckMate 057 trial was presented, along with the subgroup analyses and patient-reported outcomes.
Correlation between PD-L1 status and benefit
According to the primary analysis, OS was significantly improved by nivolumab (12.2 vs. 9.4 months with nivolumab and docetaxel, respectively; p = 0.0009), which provided a reduction in the mortality risk of 28 % [3]. Even though the Kaplan-Meier curves crossed in the beginning, the analysis clearly showed consistent benefit of the immunotherapy over the longer term [4]. At 18 months, OS rates were 39 % vs. 23 %, respectively. Also, the primary analysis demonstrated improvements in ORR (19 % vs. 12 %; p = 0.0246) and duration of response (17.2 vs. 5.6 months). Superior responses to nivolumab were also observed across almost all of the subgroups. This benefit stood out in the group of former and current smokers (ORR: 22 % vs. 11 %). Conversely, the ORR for nivolumab was slightly lower compared to docetaxel in the group of patients with positive EGFR mutation status (11 % vs. 16 %).
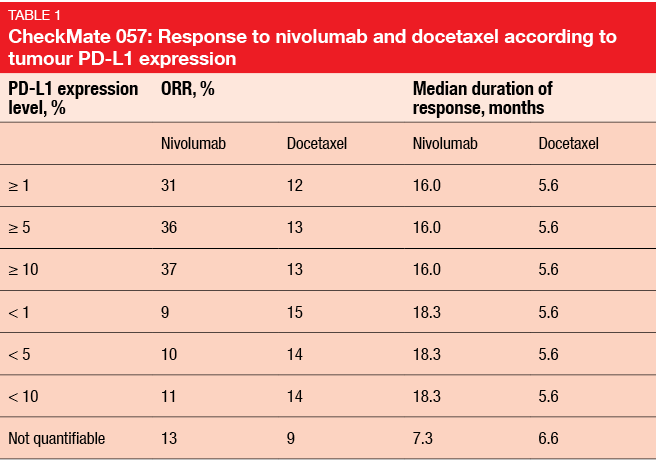
An exploratory analysis established a potential correlation between PD-L1 expression status and efficacy of nivolumab. Indeed, whereas survival appeared to be comparable in the two treatment arms in the PD-L1–negative subgroup, nivolumab-treated patients fared better than those who received docetaxel when they were PD-L1 positive. Moreover, there was a correlation between PD-L1 expression status and ORR, again favouring patients with PD-L1–positive tumours (Table 1). Duration of response, on the other hand, was not affected by PD-L1 status.
Patient-reported outcomes in CheckMate 057
Symptom burden was assessed using a pre-specified Lung Cancer Symptom Scale questionnaire, which covers the most important lung-cancer-related symptoms (e.g., fatigue, dyspnoea, pain, haemoptysis, cough, anorexia). The predefined secondary endpoint was symptom improvement rate at week 12. For this outcome, the results were comparable between the two arms, although the nivolumab therapy appeared to do better, and provided symptom stabilisation over time.
With respect to tolerability, the analysis showed that the administration of nivolumab resulted in substantially fewer AEs. This was particularly true for grade 3/4 treatment-related AEs, severe AEs, and events that led to discontinuation. However, specific immune-related toxicity, which has been described for all anti-PD-1 and anti-PD-L1 antibodies, occurred in some patients. Overall, the safety profile of nivolumab was favourable compared to that of docetaxel, and consistent with prior studies.
The authors concluded that in CheckMate 057, nivolumab continues to demonstrate superior OS versus docetaxel in pre-treated patients with advanced non-squamous NSCLC. The magnitude of benefit was greater among the PD-L1 expressors compared to the non-expressors, although there were clinical benefits in both groups.
Durvalumab plus tremelimumab
Dual immune checkpoint blockade with anti-CTLA-4 and anti-PD-1 antibodies is an established option in the treatment of melanoma. These two approaches enhance T-cell anti-tumour activity through different but complementary mechanisms. The notable effects of this strategy include particularly pronounced depth of response, which is known to correlate with improved long-term benefit. Promising activity of ipilimumab plus nivolumab in lung cancer was already obtained in the CheckMate 012 trial; here, patients with advanced NSCLC received the two antibodies in the first-line setting [5]. The combination gave rise to deep and durable responses.
Another potentially useful dyad is the anti-PD-L1 antibody durvalumab and the anti-CTLA-4 antibody tremelimumab. A non-randomised, open-label, phase Ib dose-escalation and dose-expansion trial assessed the safety and anti-tumour activity of this combination in patients with advanced NSCLC [6]. Multiple dose combinations were tested: durvalumab was administered at 3 mg/kg, 10 mg/kg, 15 mg/kg or 20 mg/kg, every 2 or 4 weeks for 26 or 13 doses, respectively; and tremelimumab was administered at 1 mg/kg, 3 mg/kg or 10 mg/kg, every 4 weeks for six doses, followed by three additional doses every 12 weeks. These treatments continued for 1 year or until disease progression. PD-L1 expression was evaluated by immunohistochemistry.
As of June 1, 2015, 102 patients have been treated in the dose-escalation phase across five centres in the US. The majority have non-squamous histology, and most are former or current smokers. More than half of these patients had already received two or three lines of treatment.
Durable responses
The grade-3/4 AE rate was 42 % in the total study population. Immune-related AEs occurred as expected, with gastrointestinal toxicity arising most frequently. Diarrhoea was observed in 32 % of the patients, and 11 % experienced grade ≥ 3 symptoms. Rash and pruritus emerged as well, but were restricted to grades 1 and 2. The increased tremelimumab dosing reduced the tolerability of the treatment. Pneumonitis, for instance, did not occur at a dose of 1 mg/kg, but showed considerable incidence at 3 mg/kg and 10 mg/kg. The majority of AEs in the tremelimumab 1 mg/kg cohort were manageable and reversible using standard treatment guidelines.
Fortunately, the tremelimumab dose of 1 mg/kg was sufficient to achieve significant anti-tumour activity. In the tremelimumab 1 mg/kg cohort, the ORR was 28 % in the total population and 44 % in the second-line patients. Responses were seen independent of PD-L1 expression, whereby patients responded even if they showed no PD-L1 expression at all. Sixty-six percent had ongoing responses at the time of the data cut-off, which indicates the durability of this treatment activity.
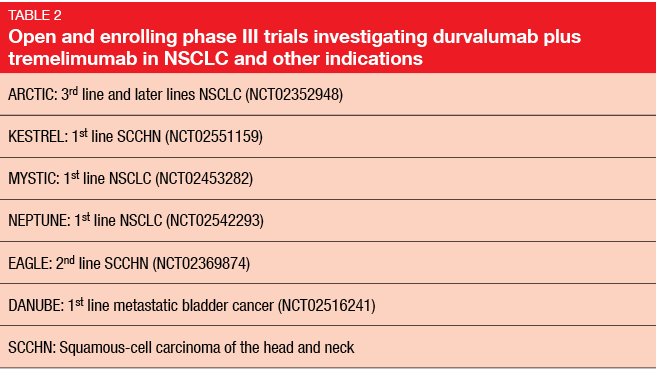
Compared to monotherapy data presented at the ASCO Congress 2015 that were obtained with durvalumab alone [7], the durvalumab plus tremelimumab combination gave rise to markedly improved ORRs. The combined treatment of durvalumab 20 mg/kg every 4 weeks with tremelimumab 1 mg/kg every 4 weeks has been selected for assessment in the phase III setting. Several phase III trials with this combination in NSCLC and other indications are open and enrolling (Table 2).
REFERENCES
- Soria J-C et al., Efficacy and safety of pembrolizumab (Pembro; MK-3475) for patients (Pts) with previously treated advanced non–small-cell lung cancer (NSCLC) enrolled in KEYNOTE-001. ECC 2015, abstract 33LBA
- Herbst RS et al., KEYNOTE-010: Phase 2/3 study of pembrolizumab (MK-3475) vs docetaxel for PD-L1–positive NSCLC after platinum-based therapy. ESMO Asia 2015, abstract LBA3_PR
- Paz-Ares L et al., Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non–squamous cell (non-SQ) non–small-cell lung cancer (NSCLC). J Clin Oncol 33; 2015 (suppl; abstr LBA109)
- Horn L et al., Phase 3, randomized trial (CheckMate 057) of nivolumab vs docetaxel in advanced non–squamous (non-SQ) non–small-cell lung cancer (NSCLC): subgroup analyses and patient-reported outcomes (PROs). ESMO Asia 2015, abstract 417O
- Rizvi NA et al., Safety and efficacy of first-line nivolumab (NIVO; anti-programmed death-1 [PD-1]) and ipilimumab in non–small-cell lung cancer (NSCLC). 16th World Conference on Lung Cancer, 2015, abstract 786
- Rizvi NA et al., Phase 1b study of the safety and antitumour activity of durvalumab (MEDI4736) plus tremelimumab in advanced NSCLC. ESMO Asia 2015, abstract 418O
- Rizvi NA et al., Safety and clinical activity of MEDI4736, an anti-programmed cell-death-ligand 1 (PD-L1) antibody, in patients with non–small-cell lung cancer (NSCLC). J Clin Oncol 33, 2015 (suppl; abstr 8032)
More posts
Immunotherapy: management of toxicity
Immunotherapy: management of toxicity The basis underlying the toxicities of im
PD-L1 expression is a nightmare in terms of complexity
PD-L1 expression is a nightmare in terms of complexity Martin Reck, MD, PhD, Dep
Immunotherapy: anti-tumour activity despite extensive pretreatment
Immunotherapy: anti-tumour activity despite extensive pretreatment The anti-PD-
Risks and chances in patients with oligometastatic disease
Risks and chances in patients with oligometastatic disease Against the backgrou
Disease progression on EGFR TKI therapy: what to do after erlotinib, gefitinib and afatinib?
Disease progression on EGFR TKI therapy: what to do after erlotinib, gefitinib and
EGFR-mutation-positive NSCLC: expanding the data pool for established treatment options
EGFR-mutation-positive NSCLC: expanding the data pool for established treatment opt