Malignant pleural mesothelioma: immunotherapy-based approaches in all treatment lines
Long-term results from CheckMate 743
The randomized phase III CheckMate 743 trial evaluated nivolumab 3 mg/kg Q2W plus ipilimumab 1 mg/kg Q6W for up to 2 years compared with cisplatin or carboplatin plus pemetrexed Q3W for 6 cycles as first-line treatment of patients with unresectable malignant pleural mesothelioma (MPM). More than 300 patients were randomized into each study arm. Overall survival (OS) constituted the primary endpoint. Indeed, the dual checkpoint inhibition significantly prolonged OS [1] and was approved as first-line treatment of unresectable MPM in many countries.
Peters et al. reported the 3-year update from CheckMate 743 at ESMO 2021 [2]. In addition to the clinical assessments, the researchers conducted exploratory biomarker analyses using a 4-gene inflammatory signature score, tumor mutational burden (TMB), and the lung immune prognostic index (LIPI). The 4-gene inflammatory signature score included the CD8A, STAT1, LAG3, and CD274 (PD-L1) genes and was established via RNA sequencing of tumor samples, while the LIPI score was based on LDH levels and neutrophil-to-lymphocyte ratio from peripheral blood.
According to the 3-year update, nivolumab plus ipilimumab continued to provide durable benefit compared to chemotherapy. OS in all randomized patients was 18.1 vs. 14.1 months, translating into a 27 % risk reduction (HR, 0.73). At 3 years, 23 % vs. 15 % of patients were alive. All subgroups benefited from immunotherapy compared to chemotherapy. Progression-free survival (PFS) remained unchanged (6.8 vs. 7.2 months; HR, 0.92), although a delayed benefit became apparent at 24 months (PFS rates, 18 % vs. 7 %) and 36 months (14 % vs. 1 %). Median duration of response was longer in the experimental arm (11.6 vs. 6.7 months) despite patients being off therapy for one year at the time of the analysis. At 36 months, 28% of responders in the immunotherapy arm had ongoing responses (vs. 0 % in the control arm).
No disadvantage after treatment discontinuation
The exploratory biomarker analysis indicated a correlation of high 4-gene inflammatory signature scores with improved survival on immunotherapeutic treatment. In the experimental arm, median OS was 21.8 vs. 16.8 months for high and low scores (HR, 0.57), and 35 % vs. 15 % of these patients were alive at 36 months. On the other hand, survival did not differ according to the 4-gene inflammatory signature score in the chemotherapy arm. Neither LIPI scores nor TMB tertiles showed a correlation with OS. After 12 additional months of follow-up, safety was consistent with the previous report. The overall rate of treatment-related AEs (TRAEs) had not changed.
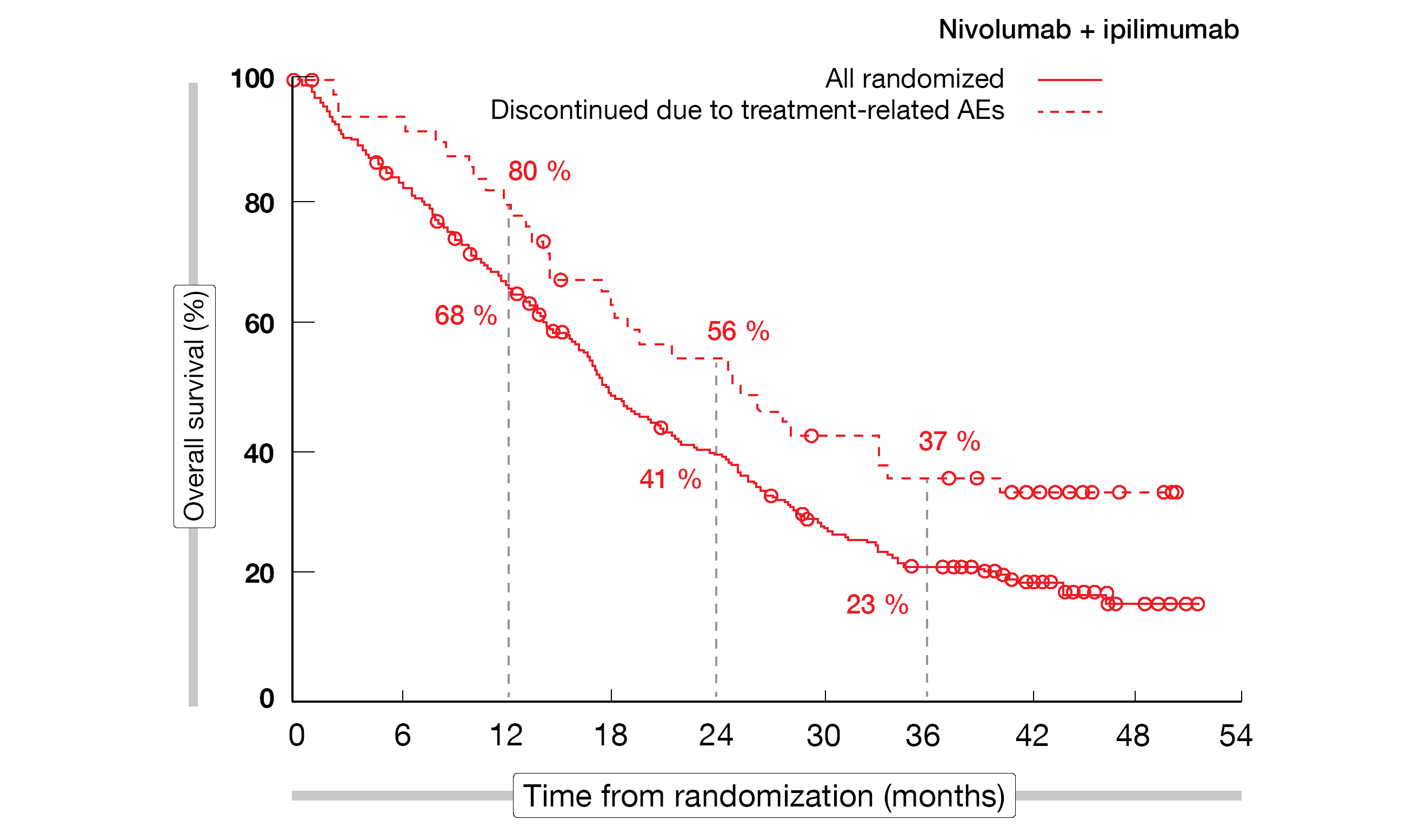
A post-hoc analysis revealed no adverse impact of discontinuation of all components of nivolumab plus ipilimumab in patients who stopped treatment due to TRAEs (Figure 1). After discontinuation, 34 % had ongoing responses for ≥ 3 years, and 37 % were alive at 36 months. In their summary, the authors pointed out that these data from CheckMate 743 confirm nivolumab plus ipilimumab as a standard of care for unresectable MPM regardless of histology.
Figure 1: CheckMate 743: overall survival in patients who discontinued first-line nivolumab plus ipilimumab compared to the total randomized population in the experimental arm
Pembrolizumab plus nintedanib in r/r disease
The phase I PEMBIB trial investigated the combined strategy of pembrolizumab and the triple angiokinase inhibitor nintedanib in patients with unresectable MPM relapsing on or refractory to platinum-based chemotherapy. Pembrolizumab 200 mg was administered Q3W, while nintedanib 150 mg was taken twice daily with a 7-day monotherapy lead-in. Ancillary analyses were performed based on blood and tumor samples. Danlos et al. reported results for 30 patients at ESMO 2021 [3]. Most patients had received one previous systemic anticancer treatment line (77 %), while 2 and ≥ 3 lines had been administered in 17 % and 6.7 %, respectively.
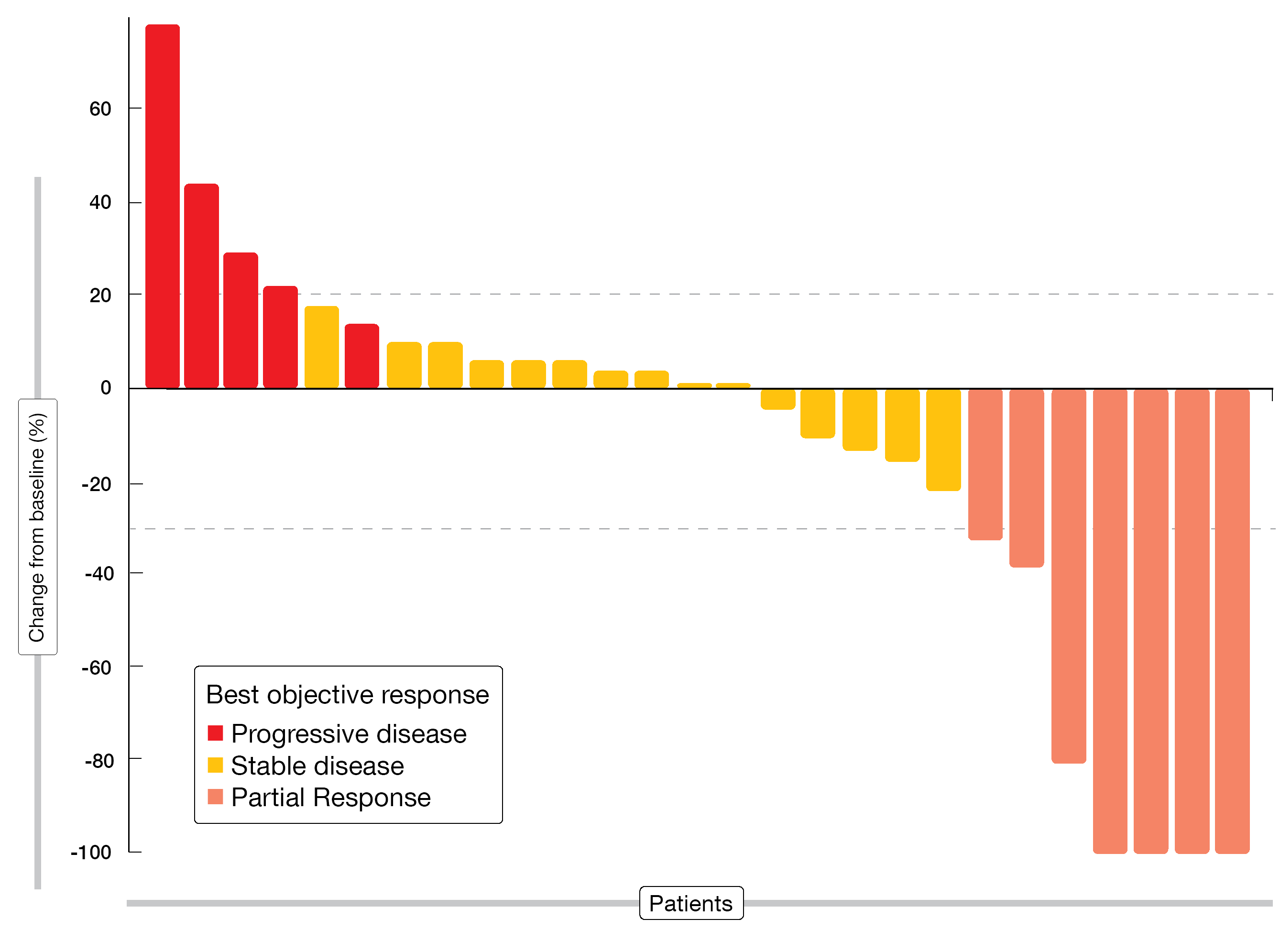
The combined treatment gave rise to a 12-week disease control rate of 68.4 % (Figure 2). Median PFS was 6.2 months. AEs proved generally manageable, with diarrhea, fatigue, and dyspnea occurring most commonly. Pembrolizumab plus nintedanib showed similar pharmacodynamic effects according to cytokine measurements. PD-L1 expression on tumor cells and tumor-infiltrating CD8+ T lymphocytes at baseline appeared to be predictive of anti-angiogenic and anti-PD-1 efficacy as these were more prevalent in patients who benefited from treatment. Furthermore, higher IL-6 concentrations showed an association with primary resistance to treatment and correlated with the global somatic copy-number alteration (SCNA) score. The authors concluded that SCNAs due to accumulation of oncogenic mutations lead to IL-6–mediated immunosuppression and resistance to anti-angiogenic and anti-PD1 therapy.
Figure 2: Responses observed with pembrolizumab plus nintedanib in patients relapsed on or refractory to previous platinum-based chemotherapy
Neoadjuvant use of atezolizumab
The S1619 study was designed based on the assumption that adding an PD-L1 inhibitor to neoadjuvant chemotherapy followed by maintenance immunotherapy after surgical resection and adjuvant radiation might increase survival outcomes [4]. Chemotherapy-naïve patients with resectable MPM received atezolizumab 1,200 mg Q3W in addition to cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 for 4 cycles. Surgery, i.e., extrapleural pneumonectomy (EPP) or pleurectomy and decortication surgery (P/D) was performed in the absence of progression. This was followed by optional radiotherapy and maintenance atezolizumab with 1,200 mg Q3W for one year. The primary endpoint was safety, tolerability, and feasibility of this approach in 24 evaluable patients.
Among 28 eligible patients, 21 completed neoadjuvant therapy. Eighteen patients with stable disease or partial response underwent surgery; P/D and EPP were performed in 17 patients and one patient, respectively. Fifteen individuals received maintenance atezolizumab. At the time of the analysis, three patients remained on this treatment. No new safety signals arose in the context of the triple regimen or the atezolizumab maintenance therapy, and no delayed treatment-related grade > 3 AEs were reported. Additional efficacy data will be updated at a future congress.
REFERENCES
- Baas P et al., First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397: 375-386
- Peters S et al., First-line nivolumab + ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year update from CheckMate 743. ESMO 2021, LBA65
- Danlos FX et al., Pembrolizumab and nintedanib for patients with advanced pleural mesothelioma. ESMO 2021, 1732M0
- Tsao A et al., S1619 A trial of neoadjuvant cisplatin-pemetrexed with atezolizumab in combination and maintenance for resectable pleural mesothelioma. WCLC 2021, OA13.01
© 2021 Springer-Verlag GmbH, Impressum