New horizons in colorectal cancer
FOLFIRINOX: real-world data in first line treatment
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide [1]
About 20 % of CRC patients are diagnosed at the metastatic stage (mCRC), with a 5-year survival rate of 14 % [2]. An AGEO (Association des Gastro-Entérologues Oncologues) multicenter real-world study investigated whether metastases resection rates and survival could be improved when adding a targeted therapy (bevacizumab or anti-EGFR agents) to the triplet-chemotherapy FOLFIRINOX in patients with mCRC; the results were presented at ESMO 2021 [3].
This retrospective study included 332 mCRC patients from 14 centers in France, who started first line treatment between January 2014 and 2019. Among them, 153 patients received FOLFIRINOX (triplet chemotherapy cohort, TC), 146 FOLFIRINOX + bevacizumab (TC-B) and 33 FOLFIRINOX + anti-EGFR (TC-E). Median age was 60.3, 59.5 and 55.1 years in the TC, TC-B and TC-E cohorts, respectively. Between the different cohorts, the primary tumor localization was significantly different (p = 0.001), with more rectal cancer in the TC cohort (39.9 %), a majority of right colon tumors in the TC-B cohort (41.1 %) and more left colon tumors in the TC-E cohort (60.6 %).
BRAF mutations were found more frequently in the TC -B group (28.5 %) compared to the TC group (8.6 %) or the TC-E group (3.1 %). RAS mutations were detected in 57.3 % and 55.9 % of the TC and TC-B groups, respectively; as expected, none were found in the TC-E group.
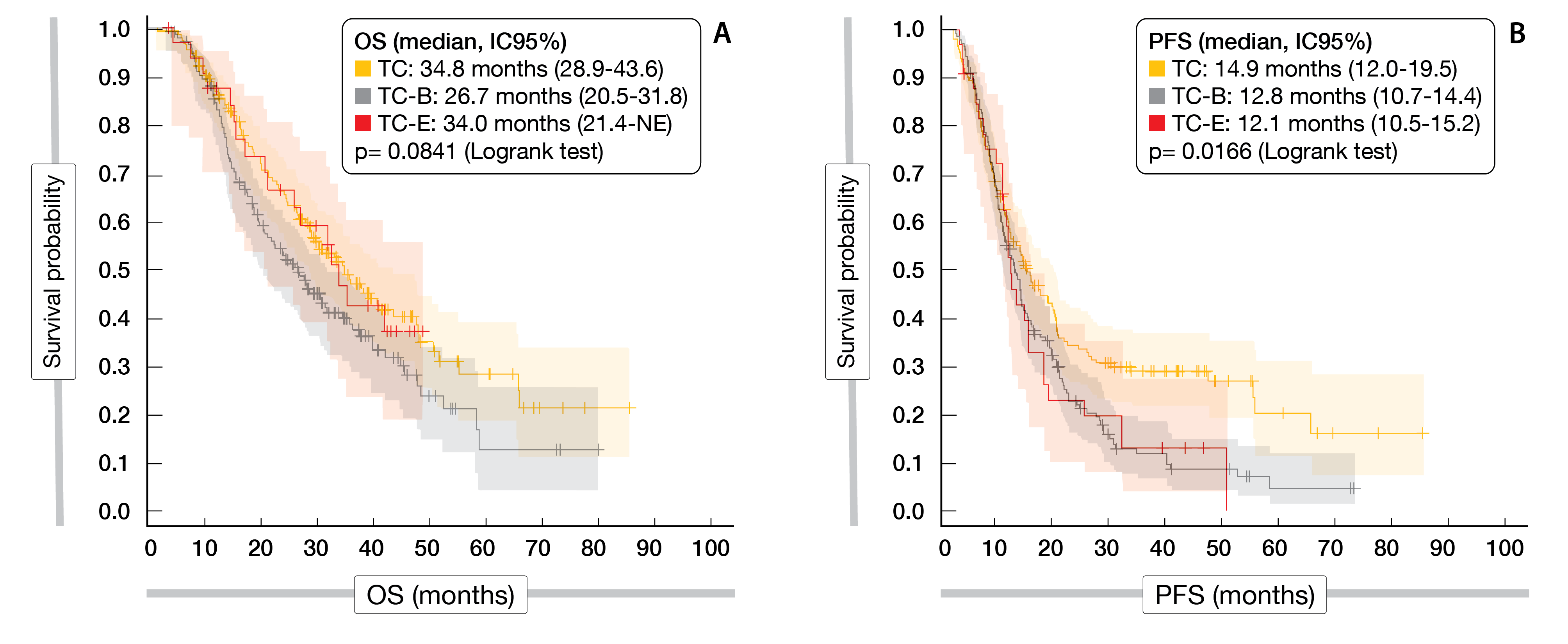
In the TC, TC-B and TC-E cohorts, the median OS reached 34.8, 26.7, and 34.0 months (p = 0.0841) and the median PFS 14.9, 12.8, and 12.1 months (p = 0.0166), respectively, (Figure 1). After adjusting for age, primitive tumor localization, number of metastasis and primitive tumor resection, overall survival (OS) and progression-free survival (PFS) were similar between the different groups. Metastasis resection rates did not differ significantly between the three investigational groups.
A subgroup analysis of BRAF-mutated patients showed a median OS of 17.9 months in the TC cohort and 13.6 months in the TC-B cohort. RAS and BRAF mutations were associated with reduced OS, while no association was observed with PFS.
Grade ≥3 adverse events (AEs) were experienced in 33.3 % (TC cohort), 27.4 % (TC-B cohort) and 34.4 % of patients (TC-E cohort).
In patients with mCRC, a similar efficacy was observed for each treatment analyzed. Considering these results in a real-world population, the authors concluded that further prospective trials are needed to explore the benefit of adding a targeted therapy to the triplet-chemotherapy FOLFIRINOX.
Figure 1: AGEO multicenter real-world study: OS (A) and PFS (B) according to treatment
MEDITREME: durvalumab and tremelimumab combined with FOLFOX
Treatment outcomes for patients with advanced CRC remain poor and new therapy options are therefore needed [4]. PD-1/PD-L1 as single immune checkpoint inhibitor (ICI) has not shown any meaningful activity in mCRC patients with microsatellite stable tumors [4]. On the other side, combined blockade with anti-PD1 and CTLA-4 antibodies demonstrated some antitumoral benefit compared with single agent PD-1 inhibitors [4, 5].
At ESMO 2021, Fumet et al. presented results of the single-arm, phase II MEDITREME study, which investigated the efficacy and safety of mFOLFOX6 (6 cycles) in combination with durvalumab (750 mg every 2nd week (Q2W)) and tremelimumab (75 mg/Q4W), followed by durvalumab maintenance therapy
in patients with previously untreated RAS-mutated mCRC (NCT03202758) [6]. Overall, 57 patients, with a median age of 63.6 years (58 % females), were enrolled. Thirty patients (52 %) had left colon or rectal cancer and 45 patients (79 %) liver metastases. In total, eleven patients (19 %) previously received FOLFOX as adjuvant therapy. Overall, 53 patients (93 %) showed KRAS mutations, four patients (7 %) were NRAS-mutated and three patients (6 %) presented microsatellite-instability high (MSI-H) tumors.
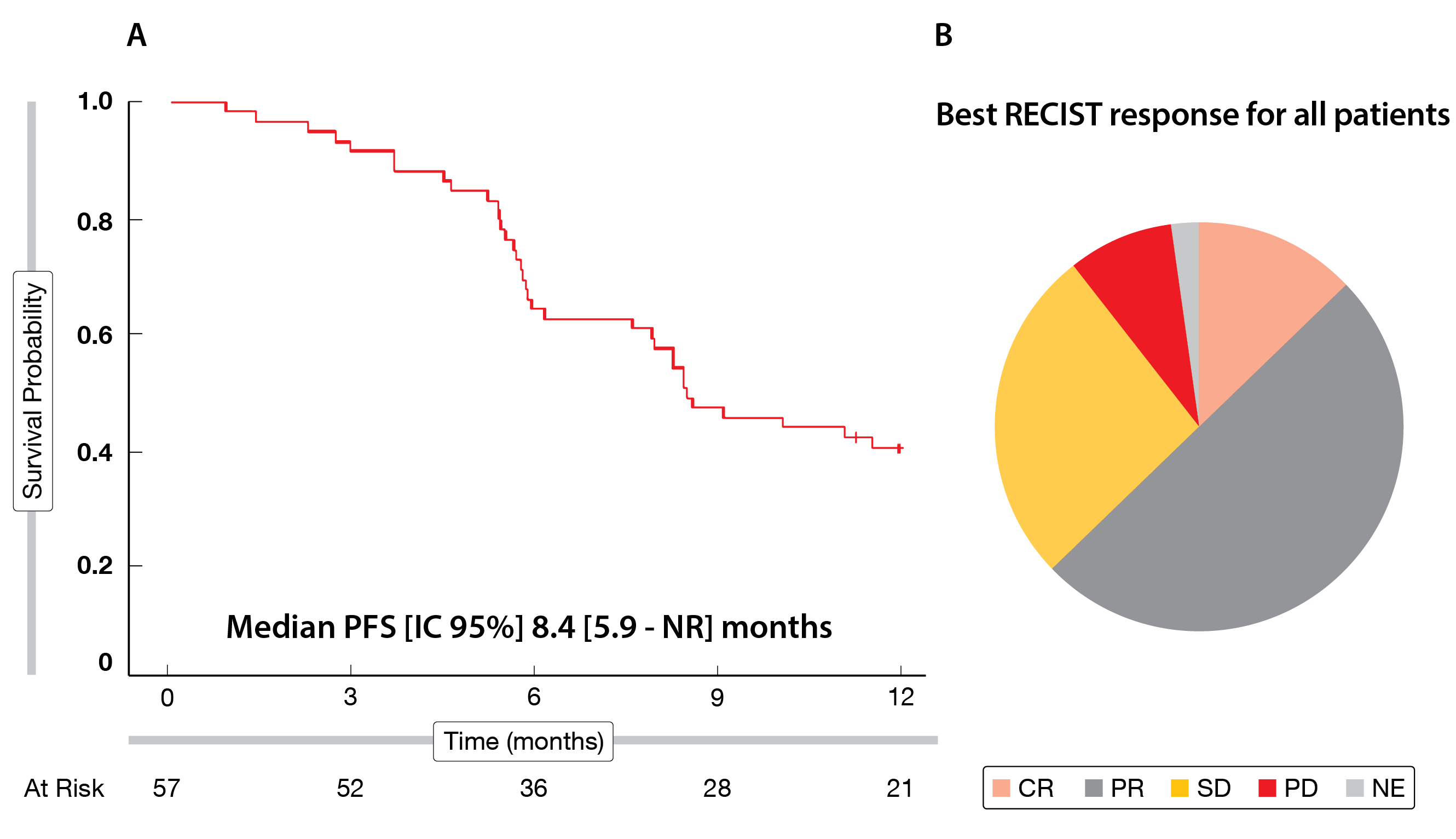
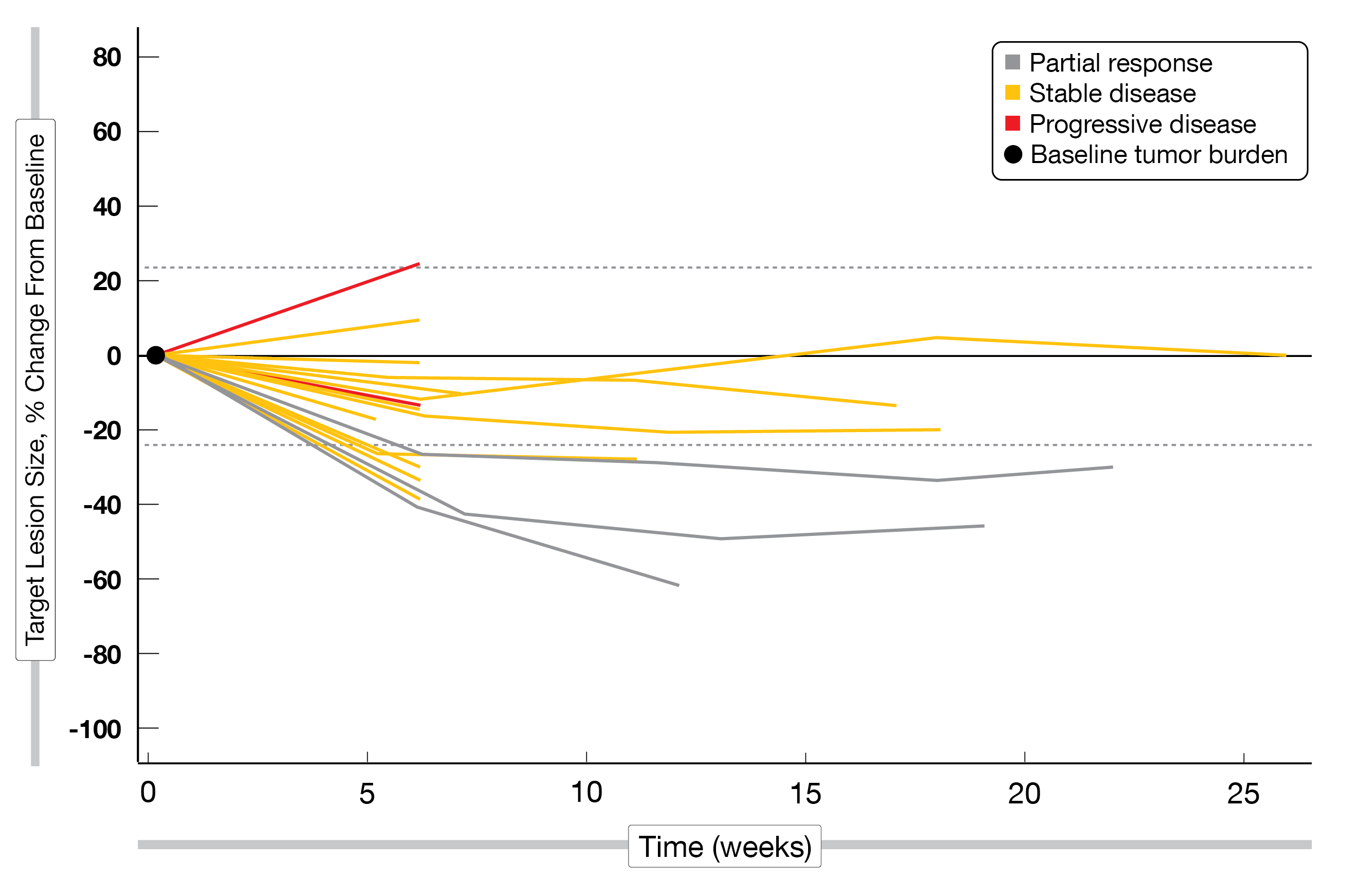
At one year follow-up, the median PFS reached 8.4 months (95 % CI, 5.9-NR) (Figure 2A); the 6-month PFS rate – the primary endpoint – attained 63.2 % (95 % CI: 49-74), and the 12-month PFS rate was 39.0 % (95 % CI: 26-51). As secondary endpoints, the objective response rate (ORR) reached 61 % and the disease control rate (DCR) was 89 %, including seven complete responses (CR), 29 partial responses (PR) and 15 stable diseases (SD) (Figure 2B). Translational analyzes showed that high baseline levels of CD4+ helper T cells (Th2) and PD-L1 positive myeloid-derived suppressor cells (MDSC) were associated with poor PFS.
Treatment-related AEs (TRAEs) of grade ≥ 3 occurred in 75 % of patients;
the most common grade 3-4 AEs experienced by patients were gastrointestinal and hematological and appear related to chemotherapy as they mainly occurred during the induction period.
Similar PFS rates were observed with this combination compared to other chemotherapy doublet plus target therapies, however, with the major advantage of only three months of chemotherapy. Further analyses are currently being performed to identify which patients might benefit most from this regimen.
Figure 2: MEDITREME trial: median PFS (A) and best response (B) according to RECIST v1.1.
CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease;
NE = not evaluable.
Trilaciclib: an innovative first in class CDK4/6 kinase inhibitor
Chemotherapy-induced damage to hematopoietic stem and progenitor cells (HSPCs) results in multi-lineage myelosuppression, which induces neutropenia, anemia and/or thrombocytopenia in treated patients [7]. Trilaciclib, a novel CDK4/6 kinase inhibitor, protects HSPCs and immune cells during chemotherapy exposure (myelopreservation) [8]. On February 12, 2021, the U.S. Food and Drug Administration (FDA) approved trilaciclib as a first in class therapy to reduce the incidence of chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy for extensive-stage small cell lung cancer (SCLC) [8]. Clinical studies in other tumor entities such as breast cancer and colorectal cancer are currently ongoing [8].
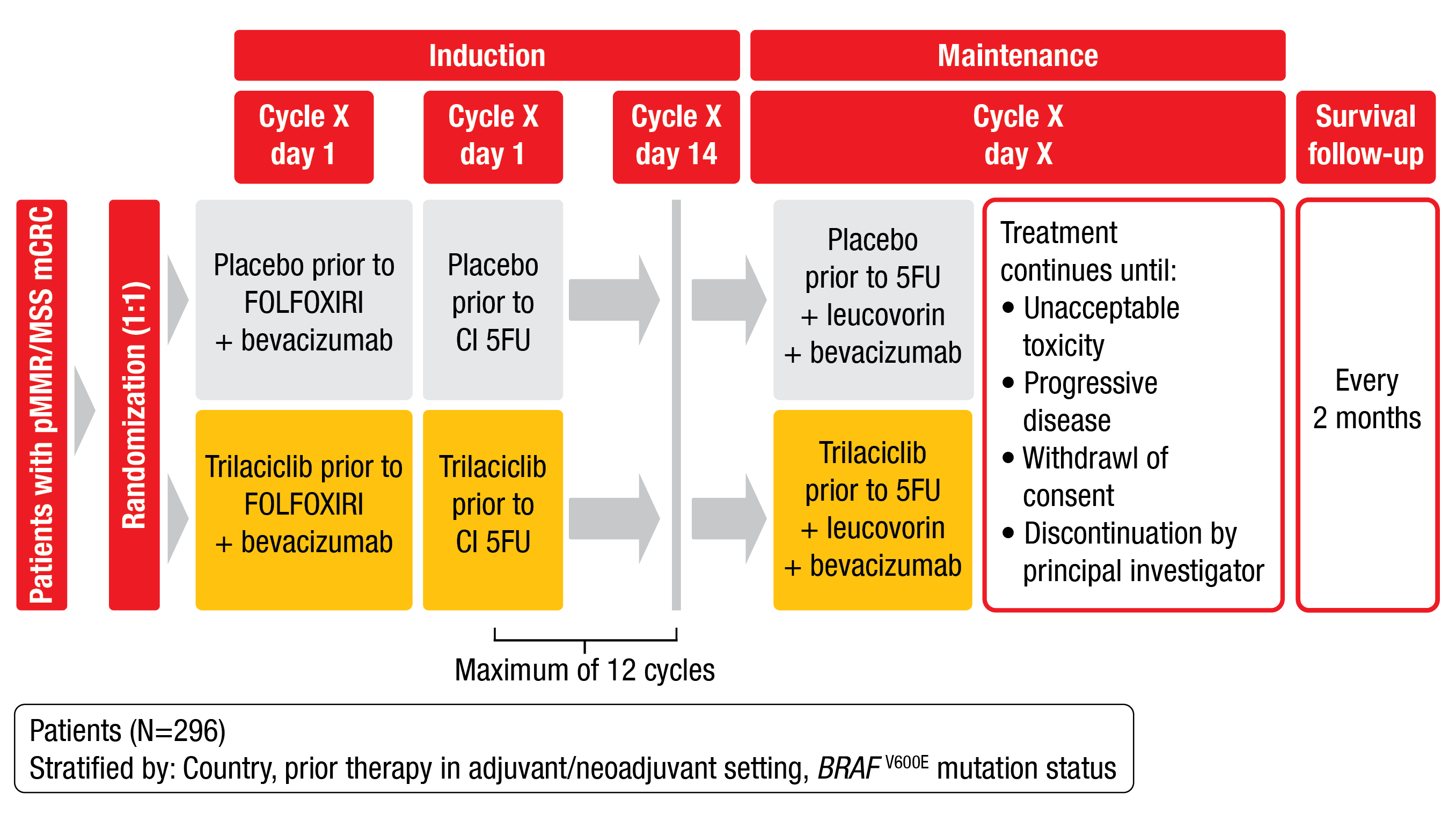
PRESERVE 1 is a randomized, phase III study (NCT04607668) evaluating the impact of trilaciclib or placebo on myelopreservation and antitumor activity when administered prior to FOLFOXIRI/bevacizumab in therapy-naïve patients with microsatellite stable (MSS) mCRC [9]. Approximately 296 eligible patients with confirmed unresectable and evaluable disease, ECOG PS ≤ 1 and adequate organ function are planned to be enrolled in 122 study locations worldwide. Exclusion criteria are prior systemic therapy for mCRC, symptomatic peripheral neuropathy, uncontrolled hypertension, or other contraindications related to FOLFOXIRI/bevacizumab treatment. Patients will be stratified by country, prior therapy, and BRAF V600E mutation status, and randomly assigned 1:1 to receive trilaciclib (240 mg/m2) or placebo on Day 1 and Day 2 prior to FOLFOXIRI/bevacizumab in 14-day cycles for up to twelve cycles (induction). Following the induction phase, patients will receive trilaciclib or placebo prior to 5FU/leucovorin/bevacizumab therapy. Primary study endpoints are duration of severe neutropenia (SN) in cycle one and occurrence of SN during the induction phase. Secondary endpoints include PFS and OS (Figure 3). The effects of trilaciclib on red blood cell and platelet lineages will also be explored. Recruitment is ongoing.
Figure 3: Study design of the phase III PRESERVE 1 trial.
LEAP-017: pembrolizumab plus lenvatinib in 2nd line mCRC
The small subset of mCRC patients with mismatch-repair-deficient (dMMR) and MSI-H derive benefit from immunotherapy, whereas the vast majority of patients with proficient MMR (pMMR) or with microsatellite stable (MSS) CRC do not [10]. The PD-1 inhibitor pembrolizumab was recently approved in Europe as first-line treatment of MSI-H or dMMR mCRC patients [11]. For patients with non-MSI-H or pMMR mCRC, the current first-line standard of care (SOC) is a chemotherapy backbone with or without VEGF and EGFR inhibitors. Thus, the improvement of survival outcomes of those patients and the circumvention of intensive chemotherapy remains an unmet need. In the previously reported phase II LEAP-005 trial (NCT03797326), the combination of pembrolizumab with the multikinase inhibitor (MKI) lenvatinib showed promising antitumor activity with a manageable safety profile [12].
At ESMO 2021, Yoshino et al. presented the study design of the phase III trial LEAP-017 (NCT04776148) evaluating the efficacy and safety of pembrolizumab in combination with lenvatinib compared to investigator’s choice of SOC therapy with regorafenib or TAS-102 (trifluridine + tipiracil hydrochloride) in patients with non-MSI-H/dMMR mCRC who have progressed on or after treatment, or have become intolerant to previous therapy [13]. Eligible criteria are the following: ≥18 years; histologically/cytologically confirmed non-MSI-H/dMMR, unresectable or metastatic stage IV (AJCC 8th edition) mCRC; ECOG PS ≤ 1. Patients will be randomly assigned to pembrolizumab (400 mg intravenously [IV], Q6W) plus lenvatinib (20 mg p.o., once daily) or to regorafenib (160 mg, once daily, Q4W) or TAS-102 (35 mg/m2, twice daily, Q4W) at investigator’s discretion. Stratification will be performed according to the absence/presence of liver metastases. OS constitutes the primary endpoint, while secondary endpoints include PFS, ORR, and DOR per RECIST v1.1 by blinded independent central review, as well as safety and tolerability. Approximately 434 patients will be globally enrolled; the recruitment is currently ongoing at 117 sites in 15 countries or regions worldwide.
Adagrasib combined or not with cetuximab by KRASG12C mutation
KRAS is the most frequently mutated oncogene in human cancer and the KRASG12C mutation occurs in up to 4 % of CRC patients; this oncogenic driver is known to be a strong negative predictive marker of cetuximab efficacy [14]. Adagrasib is a selective and irreversible inhibitor of KRASG12C, whose efficacy has already been demonstrated in non-small cell lung cancer (NSCLC) [15]. During a presentation at ESMO 2021 meeting, J. Weiss hypothesized that the combination of adagrasib and the EGFR inhibitor cetuximab may enhance the inhibition of the KRAS-dependent signaling [16].
KRYSTAL-1 was a multi-cohort, phase I/II study (NCT03785249) in patients with unresectable or metastatic solid tumors harboring a KRASG12C mutation. The recommended dose of 600 mg adagrasib twice daily, which has been set-up in the dose escalation part of the study, was then subsequently evaluated in multiple phase Ib and II expansion cohorts. Primary study endpoints included safety and clinical activity in the phase I, as well as ORR according to RECIST v1.1 in the phase II of the trial.
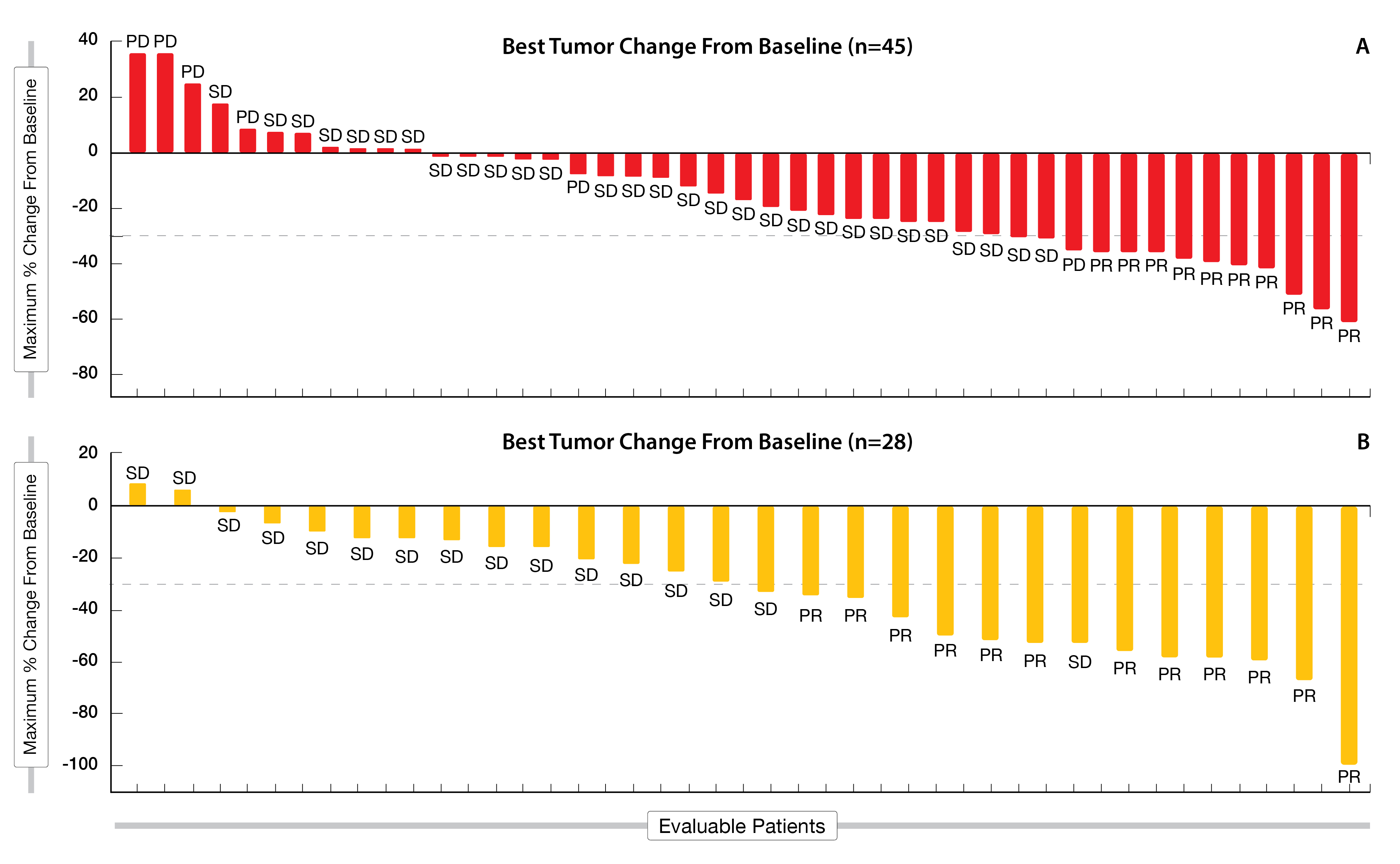
Preliminary results of adagrasib monotherapy (n = 45) or combination with cetuximab (n = 28) in heavily pre-treated KRASG12C-mutated CRC evaluable patients, who had received at least two prior lines of systemic therapies, have been presented at ESMO 2021 [16]. After a median follow-up of nearly nine months, adagrasib monotherapy resulted in an ORR of 22 % and a DCR of 87 % (Figure 4A). Additionally, among the 28 patients evaluable for clinical activity, adagrasib combined with cetuximab led to an ORR of 43 % (including 1 confirmed PR) and a promising DCR of 100 % after a median follow-up of seven months (Figure 4B). At the time of the data cut-off, data for duration of response (DoR) and PFS were still immature in the combination cohort, while 63 % of patients were still on treatment; in the monotherapy arm, median DoR was 4.2 months and median PFS reached 5.6 months.
TRAEs of any grade occurred in all study patients; grade 3-4 TRAEs were experienced by 30 % of patients treated with adagrasib alone and 16 % of patients who received the combined treatment. Those TRAEs led to treatment discontinuation in 6 % in the combination cohort versus none in the monotherapy arm.
Adagrasib showed encouraging antitumoral effects and was well tolerated whether as monotherapy or combined with cetuximab. This combination therapy is currently being evaluated as second-line treatment in the randomized, phase III KRYSTAL-10 study (NCT04793958) of patients with KRASG12C-mutated CRC.
Figure 4: Waterfall plot following adagrasib monotherapy (A) and combination (B) with cetuximab in the KRYSTAL-1 trial.
Synergistic effects of sotorasib combined to panitumumab
Sotorasib, a first in class RAS GTPase family inhibitor that selectively and irreversibly targets the KRASG12C mutation, has shown anticancer activity in solid tumors [17] and was thus FDA approved on May 28, 2021 for patients with KRASG12C-mutated NSCLC [18]. Previous data demonstrated an ORR of 7.1 % in pretreated KRASG12C-mutated CRC patients [17]. As KRASG12C blockade can lead to accumulation of upstream EGFR signaling, the combination with the anti-EGFR antibody panitumumab might act synergistically to inhibit cancer growth, as suggested by preclinical data [19, 20].
CodeBreaK 101 (NCT04185883) is an ongoing phase Ib study with a dose exploration phase (part 1) to identify a safe and tolerable daily oral dose of sotorasib (960 mg initial p.o. daily, de-escalated to 720 mg or 480 mg) plus panitumumab (6 mg/kg IV, Q2W) in patients with previously treated mCRC, and a dose expansion phase (phase 2) [21]. Cohort A (part 1) comprised patients previously treated with or naïve for KRASG12C inhibitors whereas Cohort A (part 2) included KRASG12C naïve patients. Among the 31 patients included so far in Cohort A, median age was 58 years and 67.7 % were female. Five patients (16.1 %) previously received sotorasib and the median treatment duration was 10.3 weeks.
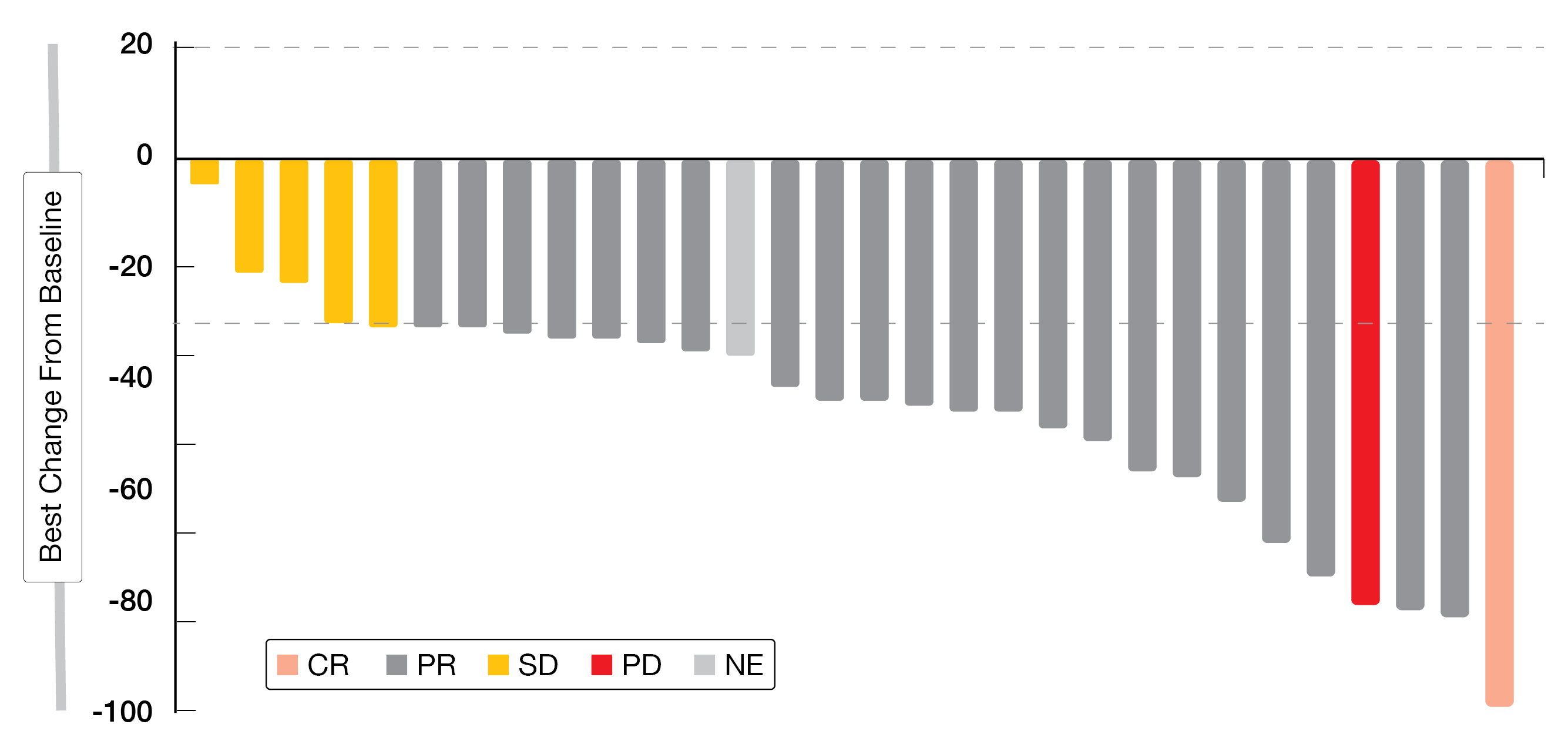
Among eight patients of Cohort A (part 1) (n = 8), DCR was obtained in 75.0 % of patients and ORR was 12.5 %, with one patient achieving a confirmed PR and five having a SD. Tumor shrinkage of 19 to 100 % was detected in two naïve patients and of 15 to 30 % in four previously treated patients. In Cohort A (part 2) (n = 18), DCR was 83.3 % and ORR reached 16.7 %, with three confirmed PRs and twelve SDs. In most patients of Cohort A (part 2), a durable decrease of target lesion size was observed (Figure 5). Overall, patients of the combined Cohort A (n = 26) achieved a DCR of 80.8 % and an ORR of 26.9 %.
No dose-limiting toxicities (DLTs) were observed. A total of 74.2 % of patients experienced TRAEs of any grade (45.2 % related to sotorasib, 74.2 % to panitumumab). Among the 12.9 % of TRAEs grade ≥ 3, dermatitis acneiform (6.5 %), dry skin (3.2 %), diarrhea (3.2 %), hypokalemia (3.2 %), hypomagnesemia (3.2 %) and rash (3.2 %) were the most common ones.
Considering the results of this early-phase study, the authors concluded that the combination therapy seems to be a safe and tolerable option showing promising efficacy in patients with KRASG12C-mutated CRC.
Figure 5: Change in target lesion size of patients in Cohort A (part 2) of the CodeBreaK 101 study.
ALTER-C002: updated results of anlotinib in RAS/BRAF wt mCRC
Anlotinib, a novel oral tyrosine kinase inhibitor (TKI) targeting mainly c-kit, PDGF receptor α and β, FGF receptor 1 - 4 and VEGF receptor 2 and 3, exerts an inhibitory effect on tumor growth and angiogenesis. It was first approved as third-line treatment for NSCLC in May 2018, followed by an approval as second-line treatment for advanced soft-tissue sarcoma in June 2019 in China [22].
The open-label, single-arm, phase II study ALTER-C002 (NCT04080843) evaluated the efficacy and safety of anlotinib in combination with capecitabine plus oxaliplatin (CAPEOX) as first-line therapy in patients with RAS/BRAF wt mCRC for which preliminary data demonstrated a high antitumor activity and a manageable safety profile [23, 24]. Patients received anlotinib (12 mg p.o., once a day at Day 1-14, Q3W), capecitabine (850 mg/m2 p.o., twice a day on Day 1-14, Q3W) and oxaliplatin (130 mg/m2 IV, on Day 1, Q3W) for six cycles, followed by anlotinib plus capecitabine maintenance until disease progression.
Updated results at the data cutoff (April 30, 2021) were presented at ESMO 2021 [25]. Among 30 patients enrolled (median age of 60 years, 13.3 % females, 86.7 % with left colon or rectal cancer, 80 % with liver metastases), 3.3 % achieved a CR, 73.3 % experienced a PR and 16.7 % had a SD (Figure 6). The ORR according to RECIST v1.1, which was defined as the primary endpoint, reached 76.7 %. Secondary outcome measures were DCR (93.3 %) and preliminary median PFS (11.4 months).
Hypertension (46.7 %), decreased neutrophil count (26.7 %) and diarrhea (13.3 %) were the most common TRAEs, while grade 3-4 TRAEs occurred in 76.7 % of patients. No extra bleeding or wound healing risk was observed during the perioperative period.
Anlotinib combined with CAPEOX provided a favorable ORR, DCR and PFS in the first-line setting of mCRC and was associated with a manageable safety profile. As a longer follow-up is needed, a phase III study has been recently launched to further assess the efficacy of this combination.
Figure 6: Waterfall plot of patients receiving anlotinib combined with CAPEOX in the ALTER-C002 trial.
Different dose schedules of cetuximab in RAS wild-type mCRC
Cetuximab is indicated for the first-line treatment of patients with EGFR-expressing, RAS wild-type (wt) mCRC in combination with FOLFOX; it is administered once a week with an initial dose of 400 mg/m2, followed by subsequent doses of 250 mg/m2 [26]. Previous studies showed the noninferiority of the off-label schedule of cetuximab (500 mg/m2, Q2W) compared with the approved schedule [27-29].
At this year’s ESMO meeting, Kasper at al. presented results from a pooled analysis of patient-level data from four studies containing information on tumor locations [30]. Patients were categorized into Q2W or Q1W subgroup based on administration regimen schedule planned at cetuximab initiation. Outcomes were assessed via logistic regression models after inverse probability of treatment weighting (IPTW), using a propensity score considering the same variables as in the main analysis, to account for differences in baseline characteristics between treatment schedules. A total of 830 and 227 patients presented with left- and right-sided primary tumor locations (PTLs), respectively. The overall ORR was 57.5 % (Q1W) and 63.6 % (Q2W), with an odds ratio (OR) of 1.292 (95 % CI: 1.031-1.617); the overall DCR reached 73.6 % (Q1W) and 78.1 % (Q2W), with an OR of 1.278 (95 % CI: 0.987-1.655). The overall resection rate of lung/liver metastases was 15.3 % (Q1W) and 20.4 % (Q2W).
In total, serious adverse events (SAEs) occurred in 29.0 % (Q1W) and 30.8 % (Q2W) of patients.
These subgroup analyses depicted no major differences between the two administration schedules (Q1W and Q2W) in terms of ORR, DCR, resection rates, or SAEs in patients with RAS wt mCRC with left-and right sided PTLS.
REFERENCES
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249
- Aparicio J et al., Metastatic Colorectal Cancer. First Line Therapy for Unresectable Disease. J Clin Med 2020; 9(12)
- Varnier R et al., FOLFIRINOX with or without targeted therapy as first line for metastatic colorectal cancer: An AGEO multicenter real-world study. ESMO 2021, 421P
- Chen EX et al., Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol 2020; 6(6): 831-838
- Overman MJ et al., Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018; 36(8): 773-779
- Fumet JD et al., Durvalumab and tremelimumab in combination with FOLFOX in patients with previously untreated RASmutated metastatic colorectal cancer: First results of efficacy at one year for phase II MEDITREME trial. ESMO 2021, 433P
- Weiss JM et al., Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol 2019; 30(10): 1613-1621
- Dhillon S, Trilaciclib: First Approval. Drugs 2021; 81(7): 867-874
- Hubbard JM et al., PRESERVE 1: A phase III, randomized, double-blind trial of trilaciclib versus placebo in patients receiving FOLFOXIRI/bevacizumab for metastatic colorectal cancer. ESMO 2021, 507 TiP
- Huyghe N et al., Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf) 2020; 8(1): 11-24
- Trullas A et al., The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. ESMO Open 2021; 6(3): 100145
- Gomez-Roca C et al., LEAP-005: A phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the colorectal cancer cohort. J Clin Oncol 2021; 39(3_suppl): 94-94
- Yoshino T et al., Pembrolizumab plus lenvatinib versus standard of care for previously treated metastatic colorectal cancer (mCRC): Phase III LEAP-017 study. ESMO 2021, 506TiP
- Rio-Vilariño A et al., Ras Family of Small GTPases in CRC: New Perspectives for Overcoming Drug Resistance. Cancers 2021; 13(15): 3757
- Veluswamy R et al., KRAS G12C-Mutant Non-Small Cell Lung Cancer: Biology, Developmental Therapeutics, and Molecular Testing. J Mol Diagn 2021; 23(5): 507-520
- Weiss J et al., KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. ESMO 2021, LBA6
- Hong DS et al., KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. NEJM 2020; 383(13): 1207-1217
- Blair HA, Sotorasib: First Approval. Drugs 2021; 81(13): 1573-1579
- Canon J et al., The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019; 575(7781): 217-223
- Lou K et al., KRAS(G12C) inhibition produces a driver-limited state revealing collateral dependencies. Sci Signal 2019; 12(583)
- Fakih M et al., CodeBreaK 101 subprotocol H: Phase Ib study evaluating combination of sotorasib (Soto), a KRASG12C inhibitor, and panitumumab (PMab), an EGFR inhibitor, in advanced KRAS p.G12C-mutated colorectal cancer (CRC). ESMO 2021, 434P
- Gao Y et al., Anlotinib as a molecular targeted therapy for tumors. Oncol Lett 2020; 20(2): 1001-1014
- Ding K et al., Efficacy and safety of anlotinib combined with CAPEOX in first-line treatment of patients with RAS and BRAF wild-type unresectable metastatic colorectal cancer – A single-arm, multi-center, phase II study (ALTER-C-002 trial). Ann Oncol 2020 (suppl 440; 469P)
- Ding K et al., Updated results of ALTER-C002: Anlotinib combined with CAPEOX in first-line treatment of patients with RAS/BRAF wild-type unresectable metastatic colorectal cancer. J Clin Oncol 2021; 39(15_suppl): e15564-e15564
- Ding K et al., A single-arm, multicenter, phase II study of anlotinib combined with CAPEOX as first-line treatment in RAS/BRAF wild-type unresectable metastatic colorectal cancer (ALTER-C002). ESMO 2021, 420P
- SMPC cetuximab. Available from:
https://www.ema.europa.eu/en/documents/product-information/erbitux-epar-product-information_en.pdf. - Brodowicz T et al., FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol 2013; 24(7): 1769-1777
- Kasper S et al., Noninferiority of cetuximab every-2-weeks versus standard once-weekly administration schedule for the first-line treatment of RAS wild-type metastatic colorectal cancer. Eur J Cancer 2021; 144: 291-301
- Rouyer M et al., Effectiveness of Cetuximab as First-Line Therapy for Patients With Wild-Type KRAS and Unresectable Metastatic Colorectal Cancer in Real-Life Practice: Results of the EREBUS Cohort. Clin Colorectal Cancer 2018; 17(2): 129-139
- Kasper S et al., Comparison of cetuximab every 2 weeks versus standard once-weekly administration for the first-line treatment of RAS wild-type metastatic colorectal cancer among patients with left- and right-sided primary tumor location. ESMO 2021, 415P
© 2021 Springer-Verlag GmbH, Impressum