Novel approaches in gastric/gastroesophageal cancer
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death worldwide [1]. Although gastroesophageal junction (GEJ) cancer, a form of gastric cancer arising in the area of the digestive tract where esophagus and stomach connect, has a lower prevalence than GC, it is continuously rising [1]. Esophageal adenocarcinoma (EAC) joins the list of malignancies as the seventh most common cancer and the sixth leading cause of death from cancer worldwide [1].
CheckMate 649: nivolumab plus chemotherapy
Cytotoxic chemotherapy has remained the standard of care in the first-line therapy of advanced or metastatic HER2-negative GC/GEJC cancer over the past decade but overall survival data are limited to less than one year [2]. Based on CheckMate 649 study results (NCT02872116), the PD-1 inhibitor nivolumab was the first immunotherapy approved by the U.S. Food and Drug Administration (FDA) for the initial treatment of patients with advanced or metastatic GC, GEJ cancer and gastroesophageal adenocarcinoma (GEA) in combination with certain types of chemotherapy [3, 4]. CheckMate 649 randomized naïve patients with advanced/metastatic GC, GEJ cancer and GEJ adenocarcinoma without known HER2-positive status into three arms (NIVO + chemo [nivolumab 360 mg plus XELOX every 3 weeks/Q3W or nivolumab 240 mg plus FOLFOX Q2W], chemo [XELOX Q3W plus FOLFOX Q2W], and NIVO + IPI [nivolumab 1 mg/kg plus ipilimumab 3 mg/kg Q3W for 4 cycles, followed by nivolumab 240 mg Q2W]). This study already showed that the addition of nivolumab led to a superior overall survival (OS), a clinically meaningful progression-free survival (PFS) benefit and a durable response in treatment-naïve patients [4]. A 24-month update of the phase III CheckMate 649 trial was presented at ESMO 2021 [2].
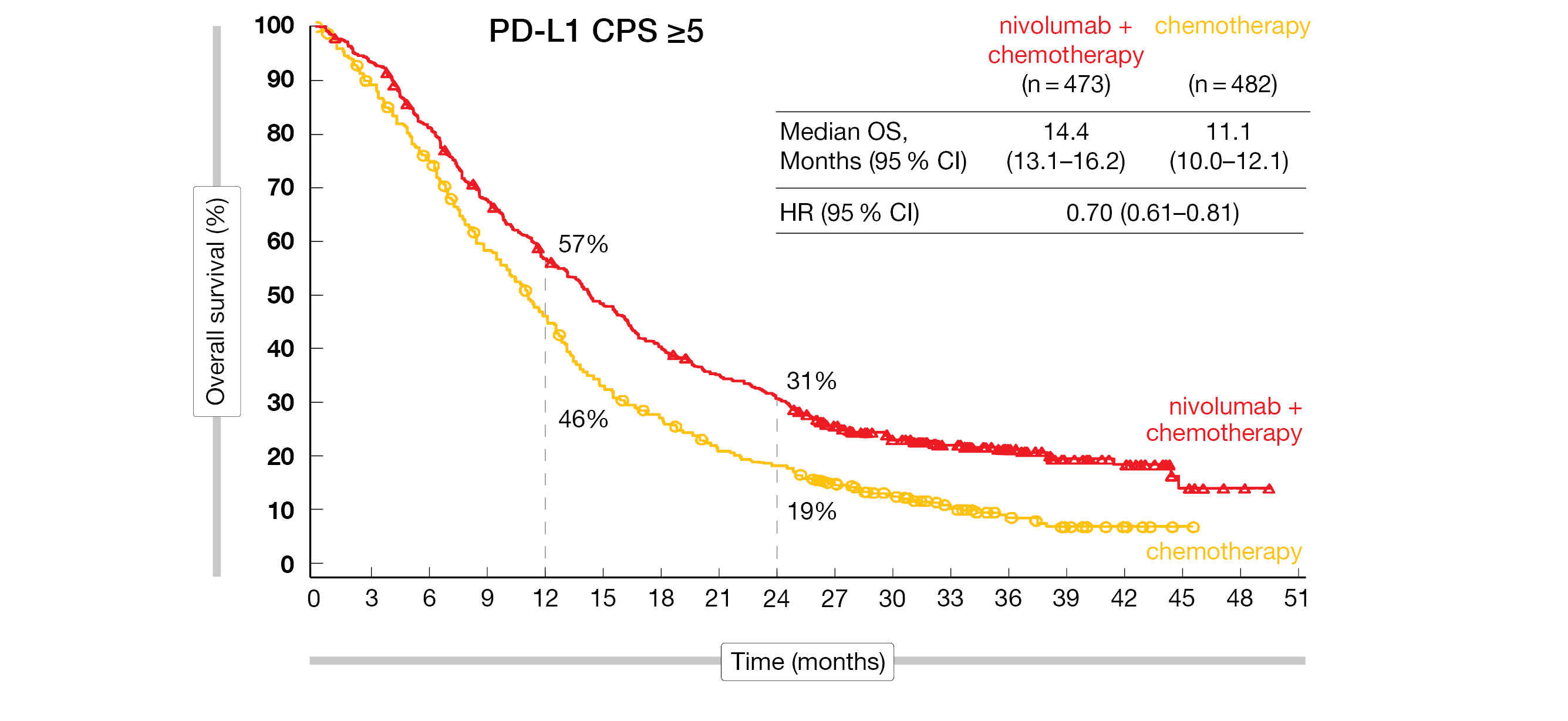
Concerning the dual primary endpoints, among patients, whose tumors expressed PD-L1 at higher levels (PD-L1 CPS ≥5), median OS reached 14.4 months with nivolumab plus chemotherapy versus 11.1 months with chemotherapy alone, while median PFS was 8.1 versus 6.1 months, respectively. nivolumab plus chemotherapy elicited a high objective response rate (ORR) of 60 % compared to 45 % with chemotherapy alone. Thirteen patients in the nivolumab plus chemotherapy arm reached a complete response (CR) and 47 a partial response (PR) versus seven CRs and 38 PRs in the chemotherapy arm (Figure 1). The median duration of response (mDoR) in the investigative and control arms were 9.7 and 7.0 months, respectively. Longer mOS and higher ORR were observed in MSI-H and MSS tumors; however, the magnitude of benefit was greater in MSI-H patients with a mOS of 38.7 months in the nivolumab plus chemotherapy arm versus 12.3 months with chemotherapy alone.
When comparing the nivolumab plus ipilumab arm with the chemo arm, no significant OS benefit for nivolumab plus ipilumab in the CPS ≥ 5 group or among all randomized patients was observed. This finding is in contrast to an OS benefit seen with the same combination in the CheckMate 648 study in esophageal squamous cell carcinoma (ESCC) [5]. Although response rates were lower with nivolumab plus ipilumab, this combination therapy did result in a longer duration of response (13.2 versus 6.9 months). Again, patients with high MSI tumors appeared to derive an advantage from the combination therapy.
No new safety signals were identified. For patients who received nivolumab plus chemotherapy, the most common grade 3 to 4 adverse events (AEs) were neutropenia (15 %), decreased neutrophil count (11 %), and anemia (6 %). In the nivolumab plus ipilumab group, patients experienced increased lipase (7 %), increased amylase (4 %) and increased ALT/AST (4% each). With chemotherapy alone, AEs included neutropenia (11-13 %), decreased neutrophil count (9-10 %), and diarrhea (3-4 %). Most immune-related adverse events (irAEs) were grade 1 or 2.
The authors concluded that the longer follow-up data of nivolumab plus chemotherapy further support its use as a new standard first-line treatment in patients with advanced G/GEJ/EA cancer.
Figure 1: CheckMate 649: overall survival of PD-L1-expressing patients with advanced or metastatic GEA treated with nivolumab plus chemotherapy or chemotherapy alone.
ORIENT-16: OS benefit of novel sintilimab
Sintilimab, a recombinant fully humanized IgG4 monoclonal PD-1 antibody, was first approved in China for the treatment of relapsed or refractory Hodgkin’s lymphoma and most recently for the first-line therapy of patients with non-squamous non-small cell lung cancer [6, 7]. As shown in preclinical data, sintilimab has a different binding site than pembrolizumab or nivolumab and showed a potentially greater affinity against PD-1 [8]. Sintilimab is currently under investigation in various solid tumor entities, including esophageal cancer [9]. At ESMO 2021, first results from a prespecified interim analysis of the randomized phase III study ORIENT-16 (NCT03745170) – evaluating sintilimab in combination with chemotherapy compared to chemotherapy alone for the first-line treatment of advanced or metastatic G/GEJ cancer – were presented [10].
As of June 20, 2021, 650 untreated Chinese patients with unresectable locally advanced or metastatic G/GEJ adenocarcinoma, regardless of PD-L1 expression, were randomized 1:1 to receive either sintilimab (3 mg/kg and 200 mg, respectively, for body weights <60 kg and ≥60 kg, IV Q3W) or placebo plus chemotherapy (CapeOX: capecitabine [1000 mg/m2 oral, twice a day, d1-14, Q3W] for up to 24 months and oxaliplatin [130 mg/m2 IV, Q3W] up to 6 cycles). Stratification factors were ECOG PS, liver metastases and PD-L1 expression.
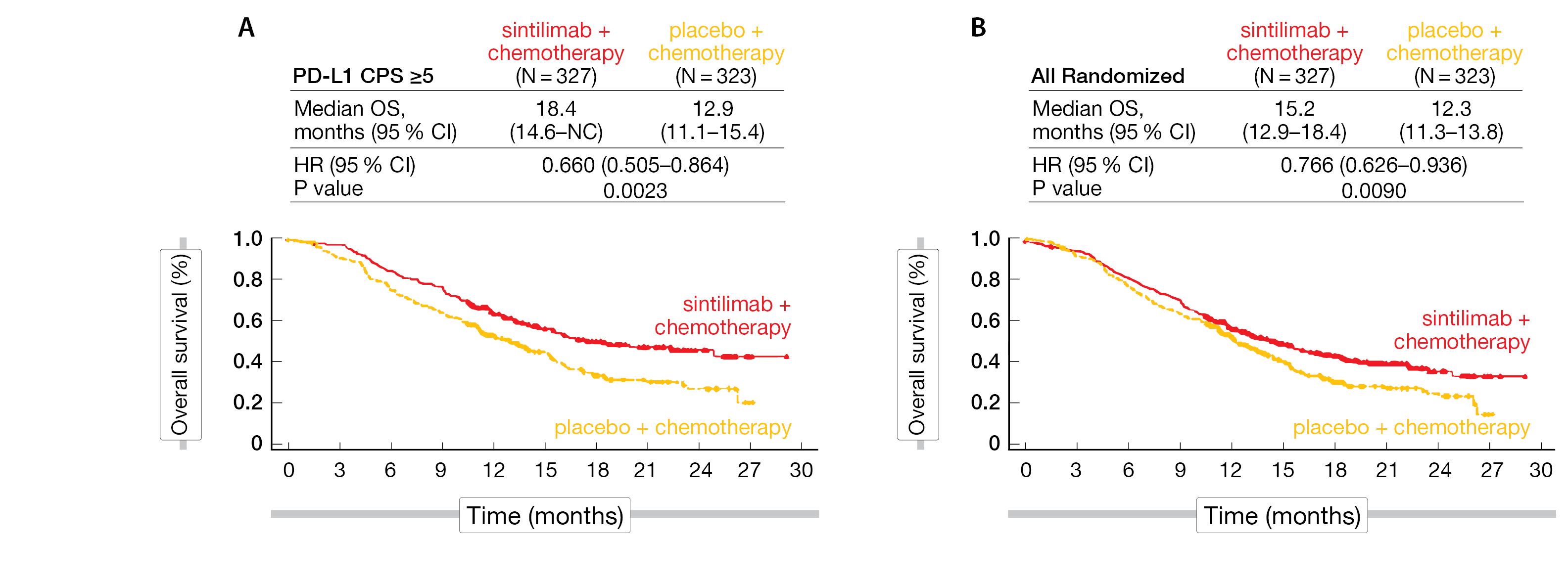
The median age of patients was 62 years in the sintilimab plus chemotherapy group compared to 60 years in the placebo plus chemotherapy group. About three quarter were male, most of the patients had GC (81 %), followed by GEJ (18 %), and 91-93 % had a metastatic disease. After a median follow-up of 18.8 months, the dual primary endpoints – OS in patients with a PD-L1 CPS ≥ 5 and in the overall patient population (ITT) – were met. Sintilimab combined with chemotherapy demonstrated superior OS compared to chemotherapy alone, with a 34 % reduction in the risk of death (HR, 0.660; 95 % CI, 0.505-0.864; p = 0.0023) and a 5.5-month improvement in the median OS (18.4 vs. 12.9 months) in patients with CPS ≥ 5; in all randomized patients, a 2.9-month improvement in mOS (15.2 vs. 12.3 months; HR, 0.766; 95 % CI, 0.626-0.936; p = 0.0090) was obtained (Figure 2). The observed OS benefit was consistent in all prespecified subgroup analyses. Similarly, median PFS – the secondary endpoint – was superior in all patients (7.1 vs 5.7 months; HR, 0.636; 95 % CI, 0.525-0.771; p < 0.0001) and those who were PD-L1 CPS ≥ 5 (7.7 vs 5.8 months; HR, 0.628; 95 % CI, 0.489-0.805; p = 0.0002).
In all patients with measurable disease, the ORR was 58.2 % versus 48.4 % in favor of sintilimab, with a median DOR of 9.8 and 7.0 months, respectively. More responders and more durable responses were seen in the sintilimab plus chemotherapy arm.
No additional safety signals were identified for the combination of sintilimab and chemotherapy. Overall, 196 (59.8 %) patients in the experimental arm and 168 (52.5 %) in the chemotherapy arm experienced grade ≥3 treatment-related adverse events (TRAEs). Six (1.8 %) fatal cases in the sintilimab group were related to TRAEs compared with two (0.6 %) in the chemotherapy group. The most common any grade TRAEs (≥ 20 %) across both investigational arms included decreased blood count parameters, anemia, nausea, vomiting, increased AST or ALT, and decreased appetite.
The authors concluded that ORIENT-16 is the first phase III trial in China to demonstrate a significant OS benefit, regardless of PD-L1 expression, and a manageable safety profile with an anti-PD-1 inhibitor combined with chemotherapy in the first-line treatment of advanced GC. The outcomes for lower PD-L1 expression (CPS <1, CPS <5 and CPS <10) were not explicitly presented; however, results shown have demonstrated improvements in patients with higher PD-L1 levels (all patients, CPS >10, CPS >5 and CPS >1), suggesting a lack of improvement in patients with lower PD-L1 levels akin to the CheckMate 649 study and other trials. Thus, data especially in low PD-L1-expressing advanced or metastatic G/GEJ cancer are awaited in the future.
Figure 2: Superior OS benefit with sintilimab plus chemotherapy in PD-L1 CPS ≥5 (A) and all randomized patients (B).
ORIENT-15: superior efficacy of sintilimab in first-line ESCC
ESCC is a histological subtype of esophageal cancer, with distinct incidence and survival patterns among races. Asian patients show a better prognosis in CSM3 mutated ESCC and a higher mutational burden with respect to TP53, EP300, and NFE2L2 [11].
The double-blind phase III ORIENT-15 study (NCT03748134) presented at ESMO 2021 enrolled 659 patients with unresectable locally advanced, recurrent, or metastatic ESCC with a ratio of 1:1 into two arms: either sintilimab (200 mg for ≥ 60 kg, 3 mg/kg for < 60 kg body weight) plus chemotherapy (TP: paclitaxel 175 mg/m2 plus cisplatin 75 mg/m2 or CF: cisplatin 75 mg/m2 plus 5-FU 800 mg/m2 on day 1-5) or chemotherapy alone [12]. Stratification factors were PD-L1, ECOG PS, liver metastases and chemotherapy.
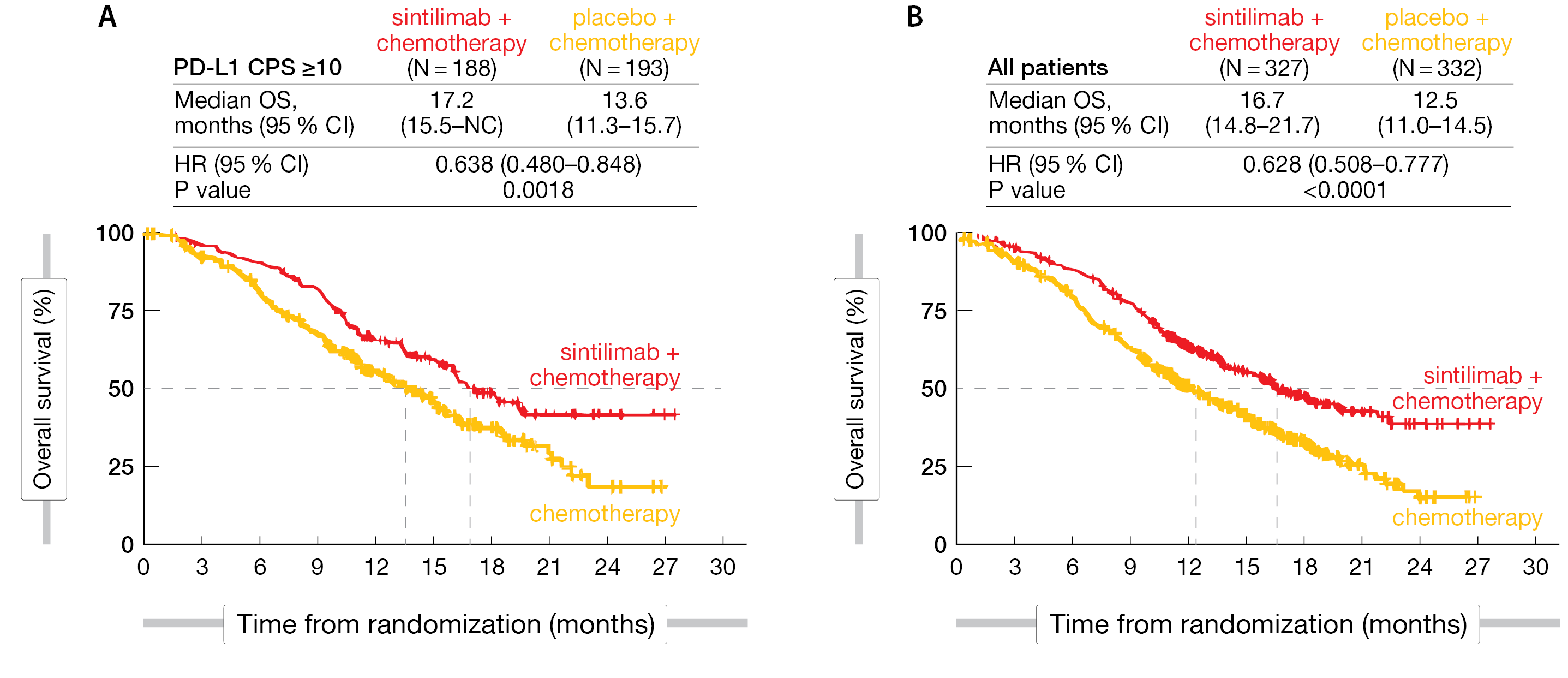
The median age was approximately 63 years, nearly all patients were Chinese, 86 % were male, and approx. 87 % had a metastatic disease. Most patients in both treatment groups had an ECOG ≤ 1. After a median follow-up of 16 months in the sintilimab plus chemotherapy and 16.9 months in the chemotherapy group, respectively, the median OS – the primary endpoint – significantly favored the experimental arm in all patients at 16.7 versus 12.5 months in the control arm (HR, 0.628; 95 % CI, 0.508-0.777; p < 0.0001). Similarly, in patients with PD-L1 CPS ≥ 10, the median OS favored the combination arm compared to the chemotherapy arm (17.2 versus 13.6 months; HR, 0.638; 95 % CI, 0.480-0.848; p = 0.0018) (Figure 3). Moreover, median PFS was superior in all patients (7.2 vs 5.7 months; HR, 0.558; 95 % CI, 0.461-0.676; p < 0.0001), as well as in those who were PD-L1-positive (8.3 vs 6.4 months; HR, 0.580; 95 % CI, 0.449-0.749; p < 0.0001). The ORR reached 66.1 % for the combined therapy versus 45.5 % in the chemotherapy arm and the median DOR was 9.7 versus 6.9 months, respectively.
Among the patients who received at least one drug dose, TRAE rates were 98.2 % in both treatment groups. Grade 3 or greater TRAE rates were 59.9 % in the sintilimab plus chemotherapy arm versus 54.5 % in the chemotherapy arm. Discontinuation because of TRAEs resulted in 20.8 % in the combination arm versus 12.3 % in the monotherapy arm, while death rates due to TRAEs were 2.8 % versus 1.8 %, respectively.
Sintilimab in combination with chemotherapy resulted in a significant OS benefit compared to chemotherapy alone in patients with advanced or metastatic ESCC, regardless of PD-L1 expression level, and thus represents a new potential first-line treatment option for this population.
Figure 3: ORIENT-15 trial: Overall survival in PD-L1 high-expressing patients (A) and in all patients (B).
JUPITER-06: toripalimab plus chemotherapy in ESCC
Toripalimab – a novel anti-PD-1 inhibitor – has been previously evaluated in a phase Ib trial in combination with chemotherapy as first-line therapy for the treatment of Asian patients with advanced or metastatic ESCC. The outcome of the randomized, double-blind phase III JUPITER-06 (NCT03829969) was lately presented at the ESMO 2021 meeting [13]. Overall, the JUPITER-06 trial has a very similar design compared to the ORIENT-15 study. PFS by a blinded independent central review per RECIST v1.1 and OS were the co-primary study endpoints.
Among the 514 analyzed patients, the addition of toripalimab to paclitaxel plus cisplatin significantly reduced the risk of death (interim OS analysis: median OS, 17.0 vs 11.0 months; HR, 0.58; 95 % CI, 0.43-0.78; p = 0.00036) and improved PFS (final PFS analysis: 5.7 vs 5.5 months; HR, 0.58; 95 % CI, 0.46-0.74; p < 0.00001) compared with chemotherapy alone.
Overall, 97.3 % of patients experienced any TRAEs in both arms; grade ≥ 3 TRAEs were 64.6 % in the combination arm and 56.0 % in the monotherapy arm. The discontinuation rate because of grade ≥ 3 TRAEs was higher in the toripalimab plus chemotherapy group (2.7 %) compared to the chemotherapy arm (0.4 %); however, fatal AEs were more often with chemotherapy alone (1.2 vs 0.4 %).
JUPITER-06 trial showed the superiority of the combination therapy compared to chemotherapy alone, independently of the PD-L1 expression level, and with an acceptable toxicity. Although the findings from this Asian study are not directly applicable to Caucasian patients, this new treatment combination has the potential to become a new standard first line therapy in patients with advanced or metastatic ESCC.
DisTinGuish: synergistic effect of innovative DKN-01
Since the 1970s, the incidence of GEA has risen considerably in Western countries [14]. Cytotoxic chemotherapy is the standard of care in the first-line therapy; as PD-L1 positivity is common in this type of cancer, immune checkpoint inhibitors (ICIs) are an option in later lines [15]. However, the low response rates and marginal improvements with ICIs in gastric or gastroesophageal junction (G/GEJ) cancers highlight the existing unmet medical need for new and effective treatments, including treatment combinations [15].
A novel approach – currently being investigated in various cancer entities – is DKN-01, a humanized monoclonal antibody that binds to and blocks the activity of Dickkopf-1 (DKK1), a secreted protein modulating the Wnt signaling pathway [16]. DKK1 plays an important antitumoral role in mediating an immuno-suppressive tumor microenvironment since overexpression of DKK1 is associated with a poor clinical prognosis [17]. In September 2020, the FDA granted DKN-01 a fast-track designation for the treatment of patients with DKK1-positive G/GEJ tumors after disease progression.
The combination of DKN-01 with the PD-1 inhibitor pembrolizumab has previously demonstrated anticancer activity in pretreated GEA patients, while high tumoral DKK1 expression was associated with longer PFS [18]. The ongoing phase II trial DisTinGuish (NCT04363801) evaluates the synergy of DKN-01 with the anti-PD-1 antibody tislelizumab in a first- or second line setting with or without chemotherapy in patients with advanced GC or GEJ adenocarcinoma; preliminary data from the first-line cohort were presented at ESMO 2021 [18].
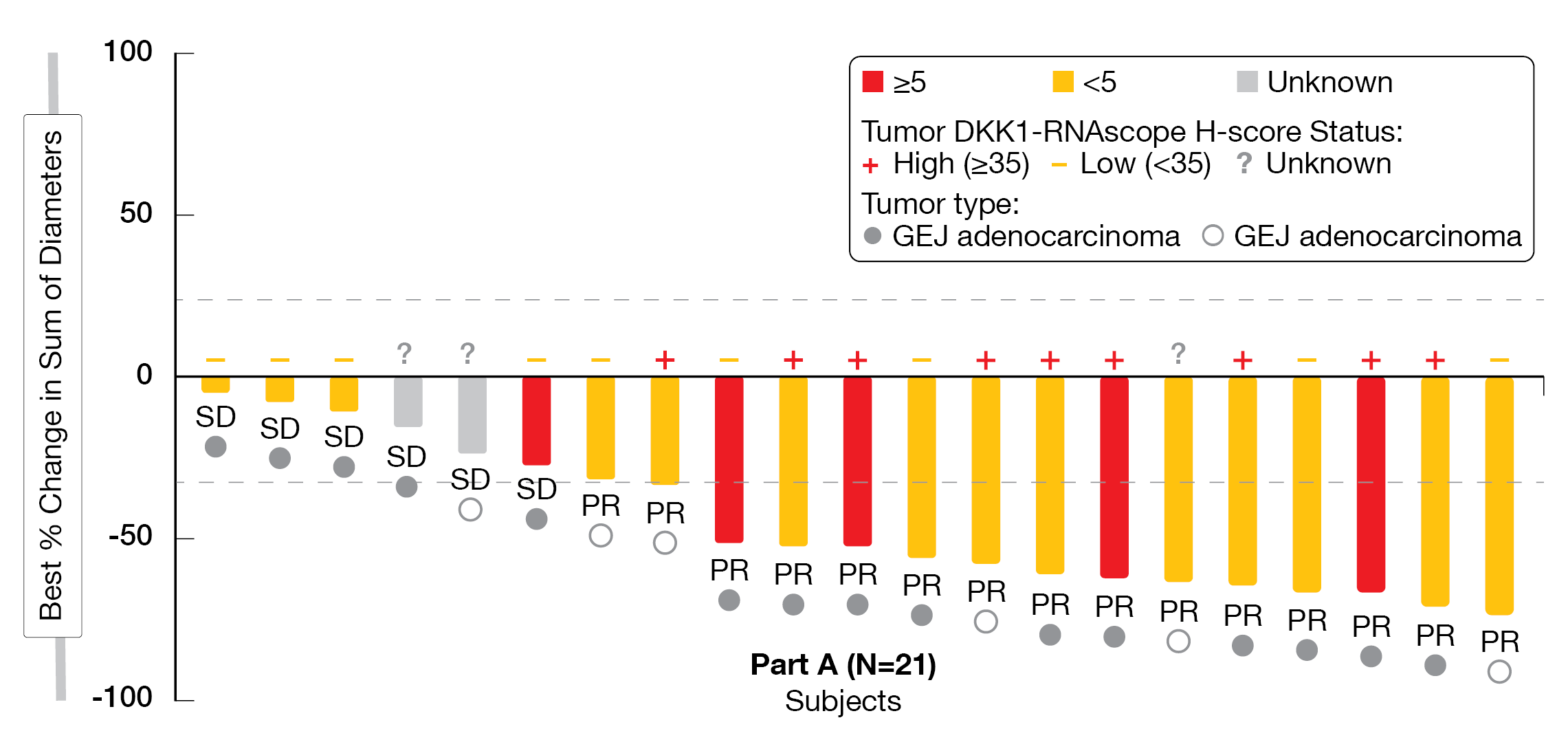
A total of 25 patients were enrolled with a median age of 61 years, 76 % of patients were male, 68 % of them suffered from GEJ adenocarcinoma and 32 % from GC, and 21 patients had tumoral DKK1 mRNA expression available, of whom 57 % were DKK1-high (8 GEJ, 4 GC) and 43 % DKK1-low (7 GEJ, 2 GC). After a median follow-up of five months, the ORR – the primary endpoint – reached 68 % including 15 PR and six SD, while the disease control rate (DCR) was 96 %. Patients whose tumors were DKK1-high showed the highest response rates (DKK-1 high, ORR 90 % versus DKK-1 low, ORR 56 %), and responses were independent of PD-L1 expression (Figure 4). PFS and DoR data were not mature yet and expected in the first half of 2022.
The DisTinGuish trial showed a manageable safety profile; DKN-01-related TRAEs occurred in 56 % of patients with fatigue being the most common one (32 %). Five patients out of 25 individuals in the overall population experienced DKN-01-related TRAEs grade ≥3: pulmonary embolism (2), diarrhea (1), decreased neutrophil count (1), and decreased blood phosphorus (1). Two patients had serious AEs related to DKN-01, one patient required a dose reduction and three discontinued the DKN-01 therapy.
Overall, DKN-01 in combination with tislelizumab plus chemotherapy was well tolerated and demonstrated a compelling ORR as a first-line treatment for advanced G/GEJ cancer.
Figure 4: DisTinGuish Trial: best overall response by PD-L1 and DKK-1 Expression. CPS:
visually-estimated combined positive score of PD-L1.
Adjuvant tislelizumab in resected ESCC
Esophageal cancer (EC) is the sixth leading cause of cancer death worldwide, with ESCC being the most common subtype globally [19].
Chemoradiotherapy alone or chemoradiotherapy followed by surgery is a widely used standard of care for patients with ESCC but recurrence rates are high after a local therapy [20]. Recent data indicate that adjuvant immunotherapy might serve as a promising new treatment option in EC-patients with residual disease found at surgery after preoperative chemoradiotherapy [21].
At ESMO 2021, Kang et al. presented the study design of the randomized phase III trial AIRES/NCCES02 (ChiCTR2100045651), which aims to compare the efficacy and safety of postoperative chemotherapy combined with the anti-PD-1 antibody tislelizumab versus tislelizumab alone (for one year) in patients with resected ESCC at high risk for recurrence [22]. Key eligible criteria are the following: ≥ 18 years, ECOG PS ≤ 1, ESCC confirmed by histology, clinical stage II-IVA (stage II only includes cT2N1M0), nodal positive disease following neoadjuvant chemoradiotherapy or chemotherapy plus surgery or upfront surgery with R0 resection. Eligible patients will be randomly assigned to receive cisplatin-based doublets Q3W for two cycles, followed by tislelizumab (200 mg intravenously (IV), Q3W) for one year or tislelizumab (200 mg IV, Q3W) alone. Stratification will be performed according to the PD-L1 expression level, preoperative induction therapy, and postoperative infectious complications. Disease free survival (DFS) constitutes the primary endpoint, while secondary endpoints include OS, as well as safety and tolerability. Exploratory endpoints will investigate distant metastasis free survival and predictive biomarkers for AEs and recurrence. Approximately 220 patients will be enrolled in China; recruitment has started in May 2021.
INTEGRATE IIb: regorafenib + nivolumab
Tumor angiogenesis has been identified as a therapeutic target in GC. VEGF, a critical regulator of pathologic angiogenesis, is expressed in tumor tissue and peripheral blood. Regorafenib – a multikinase inhibitor (MKI) targeting VEGF, TIE-2, PDGF-β, RAF, RET and KIT – has shown efficacy in advanced GC; recent data demonstrated promising synergistic effects when combined with ICI [23, 24].
The ongoing phase III trial INTEGRATE IIb (NCT04879368) evaluates the impact of regorafenib combined with nivolumab in pretreated G/GEJ cancer [25]. Eligible patients have a metastatic or locally recurrent gastroesophageal cancer which has arisen in any primary gastroesophageal site (gastroesophageal junction or stomach) with adenocarcinoma or undifferentiated carcinoma histology, and with a minimum of two lines of prior anticancer therapy (at least one platinum agent and one fluoropyrimidine analogue). Exclusion criteria include prior VEGF TKI treatment (anti-VEGF monoclonal antibody treatement is permitted), bleeding disorders, uncontrolled CNS/brain metastases, and abnormal thyroid function. Patients will be stratified by geographic tumor region, prior VEGF inibition and prior immunotherapy, and randomly assigned 2:1 to receive regonivo (Regorafenib 90 mg orally, once daily on days 1-21 of each 28 day cycle; Nivolumab 240 mg IV, every 2 weeks) or a chemotherapy of investigator’s choice (paclitaxel, docetaxel, irinotecan, or oral trifluidine/tipiracil).
Primary study endpoints are OS, while secondary endpoints include PFS, response rate, quality of life, toxicity, and exploratory correlative biomarkers. Approximately 450 adult patients are planned to be enrolled in 75 study locations in the U.S., Australia, Canada, Japan, Korea, New Zealand and Taiwan.
Novel anti-PD-1 pucotenlimab in second line G/GEJ cancer
Pucotenlimab (HX008) is a novel highly selective humanized anti-PD-1 antibody with a S228P hinge mutation and an engineered Fc domain. Pucotenlimab exhibits a decreased antibody-dependent cellular cytotoxicity (ADCC) and a complement-dependent cytotoxicity preventing depletion of PD-1-expressing lymphocytes, while retaining their antitumor activity [26, 27].
Phase I and II studies revealed durable antitumor activity of pucotenlimab in combination with chemotherapy in the first- and second line settings of G/GEJ cancer [28, 29]. Huang et al. presented at this year’s ESMO meeting the study design of a currently ongoing randomized phase III trial (NCT04486651); this study is investigating the efficacy and safety of pucotenlimab plus irinotecan as 2L therapy in patients with advanced G/GEJ adenocarcinoma who have progressed after failure of the first-line treatment with platinum and/or fluoropyrimidine therapy [30]. Key inclusion criteria include histologically or cytologically confirmed unresectable or metastatic G/GEJ adenocarcinoma, ECOG ≤ 1, and adequate organ and hematopoietic functions. Stratification will be performed according to ECOG PS, PD-L1 expression, and time to progression from first line treatment. Eligible patients are randomized to receive pucotenlimab (200 mg IV Q3W) plus irinotecan (160 mg/m2 IV, Q2W) or placebo plus irinotecan (160 mg/m2 IV, Q2W). OS will be primarily analyzed, while PFS, ORR, DCR, DOR and OS in PD-L1 CPS ≥ 10, as well as safety will be secondarily evaluated. The trial is currently recruiting patients in 64 Chinese centers.
Early results of zanidatamab in HER2-positive GI tumors
Zanidatamab (ZW25) is a novel bispecific antibody directed against HER2, that can simultaneously bind two non-overlapping epitopes, resulting in HER2 signal blockade [31]. Previously published data have shown promising and durable antitumour activity, as well as good tolerability, in patients with heavily pretreated advanced or metastatic HER2-expressing GEA or in G/GEJ adenocarcinoma [32].
The multicenter phase II trial (NCT03929666) was presented at ESMO 2021; this study investigated the safety, tolerability and anticancer activity of zanidatamab (30 mg/kg or 1800/2400 mg IV, Q3W) plus standard first-line combination chemotherapy in HER2-expressing GI tumors among patients in USA, Canada and Korea [33]. Of the 36 eligible patients, the primary tumor location was esophageal (n = 9), GEJ (n = 14), and gastric (n = 13); 89 % of patients were male, and 89 % showed HER2-positivity. Patients in the zanidatamab plus CAPOX group (n = 12) achieved a confirmed ORR (cORR) of 92 %. Eleven patients had a PR, and one patient a stable disease (SD), while mDoR was not reached (NR). Patients (n = 2) in the zanidatamab plus FP (5-FU and cisplatin) arm reached a cORR of 100 % with two CRs (mDoR, NR), while patients in the zanidatamab plus mFOLFOX6 cohort (n = 14) experienced a cORR of 57 % with one CR, seven PRs , three SDs and three progressive diseases (PDs), with a mDoR of 16.4 months. Across all treatment regimens, the cORR was 75 % with a mDoR of 16.4 months. With a median follow-up of 6.9 months, the mPFS reached twelve months and 61 % of patients were still on zanidatamab treatment.
Treatment-emergent adverse events (TEAEs) were observed in 69 % of patients (n = 36) and consistent with previous reports. Diarrhea – the most frequent TEAEs (42 %) across treatment regimens – was manageable in the outpatient setting and mitigated by prophylaxis. No severe infusion related reactions or cardicac events were observed.
Based on these results, a global phase III study (HERIZON-GEA-01) will start its enrollment in 2021 to evaluate zanidatamab plus chemo (CAPOX or FP) in combination with the PD-1 inhibitor tislelizumab for the first-line treatment of HER2-positive GEA.
Margetuximab combined to retifanlimab in HER2+ GEJ cancer
Margetuximab, a chimeric, Fc-engineered, monoclonal antibody targeting the same epitope as trastuzumab, was approved in December 2021 by the U.S. FDA for pretreated HER2-positive patients with metastatic breast cancer [34]. Retifanlimab (also known as MGA012) is an investigational anti-PD-1 antibody being developed to be used either as monotherapy or in combination with other potential cancer therapeutics [35].
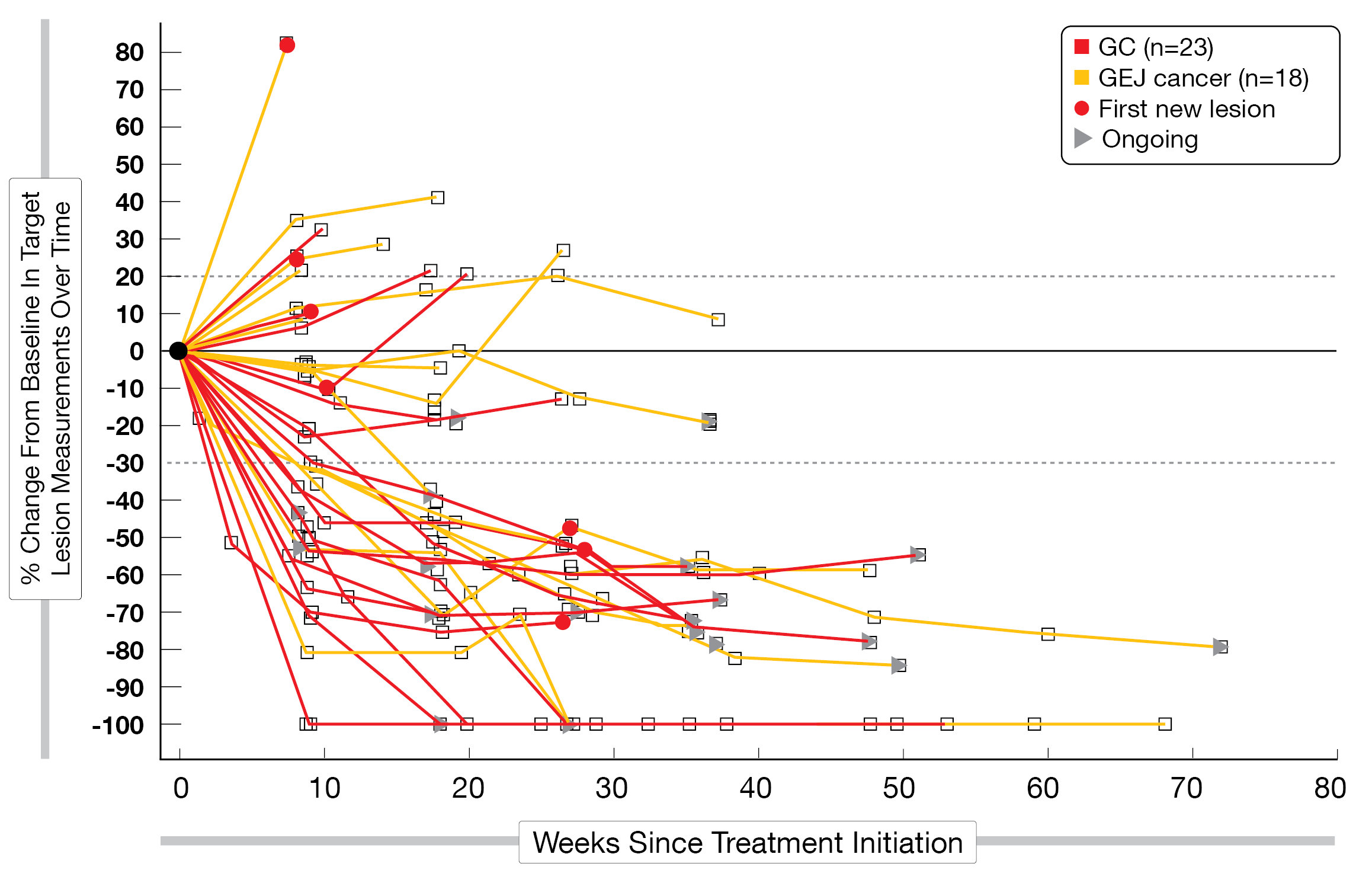
The combination of both agents, margetuximab and retifanlimab, is currently evaluated in the phase II/III MAHOGANY trial (NCT04082364) for the treatment of naïve patients with advanced GEJ adenocarcinoma [35]; first results of the cohort A were presented at ESMO 2021 [36]. This interim analysis included 43 patients (HER2-positiv, PD-L1 CPS ≥1) enrolled with gastric cancer (58.1 %) and GEJ cancer (41.9 %); most of them were male (90.7 %) and had a metastatic disease (83.7 %). Patients with central nervous system metastases were excluded. The best overall response (BOR) assessed by an independent review committee for the first 40 response-evaluable non-MSI-H patients was 52.5 % (n = 21). Four patients achieved a CR, 17 patients showed a PR, nine patients experienced a SD, and eight patients had a PD (Figure 5). The mDoR was 10.3 months, while the mPFS reached 6.4 months, with a 12-month PFS rate of 50 %, and mOS was not reached (18-month OS rate, 85 %).
The combination was generally well tolerated. Treatment-related adverse events (TRAEs) occurred in 81.4 % of patients, mostly fatigue (21 %), infusion-related reaction (19 %), rash (19 %), diarrhea (16 %) and pruritus (16 %). Overall, 18.6 % of TRAEs were grade 3 to 4. To note, three patients discontinued the combination therapy because of irAEs (grade 3 renal function, grade 3 hepatitis and grade 1 diabetic ketoacidosis one each).
These study outcomes indicated that simultaneous targeting of HER2 and PD-1 (margetuximab plus retifanlimab) may be a potential option for the first-line therapy of HER2-positive patients with GEA.
Figure 5: MAHOGANY trial: change in tumor size over time.
Synergistic effects of PD-L1 and CTLA4 inhibitors
Combinations of ICI have shown encouraging progress in the treatment of various human cancers; however, the higher costs and greater side effects of such immune combinations compared with single-agent immunotherapies may limit their further applications [37]. Previous studies suggested potential synergetic effects of an immunotherapy combination in HER2-positive GEA patients [38].
KN046 – a novel bispecific antibody directed against PD-L1 and CTLA-4 – is delivered safely and effectively via a smart nano-delivery agent (ZIF-8) directly to the tumor area. KN046 was tested in combination with KN026, a novel bispecific antibody that simultaneously binds to two distinct HER2 epitopes. At ESMO 2021, preliminary results of a phase Ib dose escalation study (NCT04040699) with KN026 + KN046 in HER2-positive (IHC 3+ or HER2 gene amplification) patients with GI tumors were presented [39].
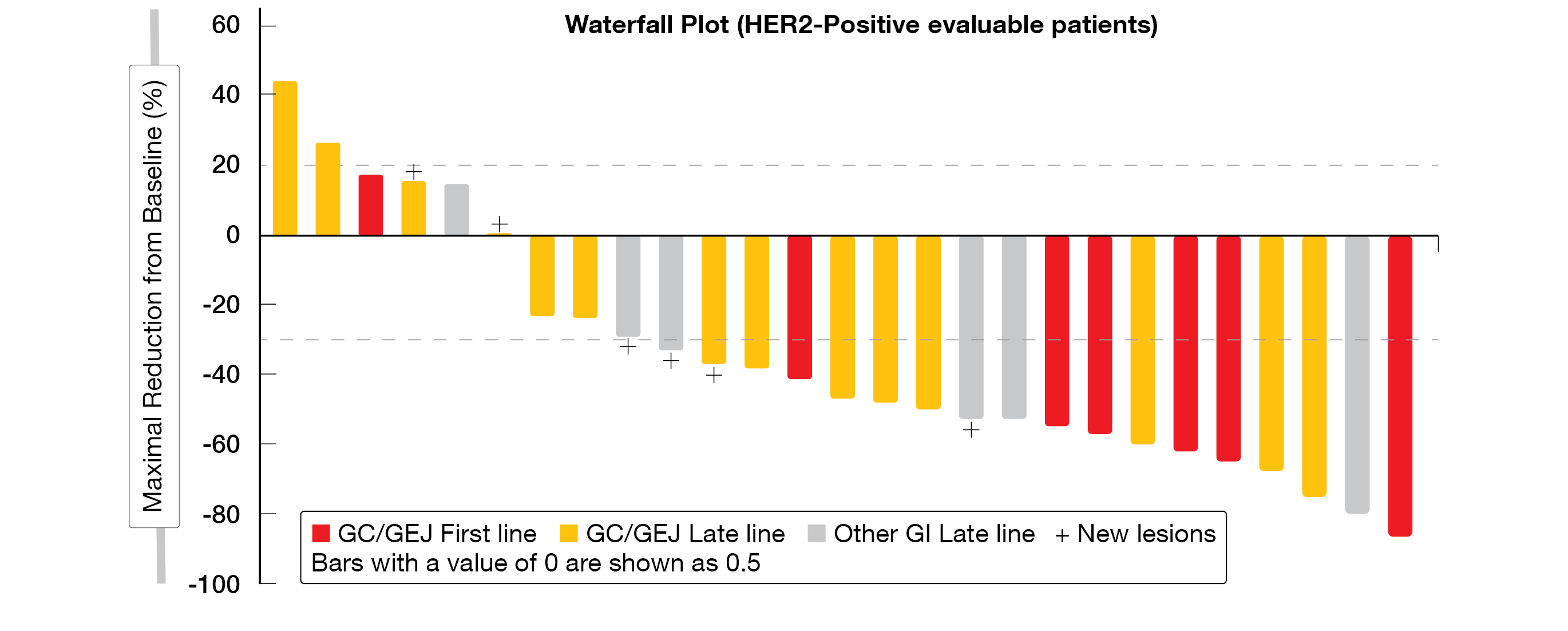
At the time of data cutoff (May 8, 2021), the dose-escalation and expansion study assigned 44 patients (68 % male, 77 % HER2-positive, 48 % previously HER2 treated) into four cohorts with different dose levels of KN026 and KN046 according to the study scheme. ORR reached 52 % (n = 27) in the overall HER2-positive group (GC/GEJ and other GI cancer), 71 % in the first-line G/GEJ cancer group (n = 7) and 43 % in ≥ 2 lines therapy G/GEJ cancer group (n = 14); DCR resulted in 85, 86 and 79 %, respectively (Figure 6). The mDoR was eleven months in both overall HER2-positive group and ≥ 2 lines therapy G/GEJ cancer groups, and not estimable in the first-line cohort. In the overall group (n = 34), the PFS reached six months, 6-month PFS-rate (PFS-6m) was 40 % and 6-month OS-rate (OSR-6m) 93 %. In the first-line G/GEJ cancer group (n = 7), PFS was not reached, while PFS-6m and OSR-6m were 86 % and 100 %, respectively. The later lines G/GEJ cancer group (n = 17) achieved a PFS of four months, with a PFS-6m of 46 % and an OSR-6m of 93 %.
In total, 91 % of patients (n = 44) experienced TEAE; anemia (38.6 %), infusion related reaction (36.4 %), increased AST (27.3 %) and diarrhea (27.3 %) were the most commonly reported TEAEs. Grade ≥ 3 TEAEs occurred in eight patients, and the most common was anemia (4.5 %).
The combination of two different ICIs in combination with HER2-positive status emerged as a promising chemo-free regimen showing clinical efficacy and manageable side-effects. Pivotal trials in HER2-positive GC/GEJ patients are planned.
Figure 6: Waterfall plot of HER2-positive patients with GI tumors treated in first or later lines.
T-Dxd in HER2-positive G/GEJ cancer
About 20 % of advanced G/GEJ cancers show overexpression of human epidermal growth factor receptor 2 (HER2) [40]. Approved targeted therapies include anti-HER2 antibody trastuzumab in combination with chemotherapy in the first-line setting and VEGFR2 inhibitor ramucirumab in combination with paclitaxel as second line therapy. Acquired resistence and decreased HER2 expression remain a challenge, as well as the limited efficacy of ICI in this population [40, 41].
Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate that delivers cytotoxic chemotherapy to cancer cells via a topoisomerase I inhibitor “payload” through a tetrapeptide-based linker attached to a HER2 monoclonal antibody binding to a specific target expressed on cancer cells. T-DXd is approved for pretreated HER2-positive advanced or metastatic GC in the US and Japan. Previous data from the DESTINY-Gastric01 trial demonstrated a clinically relevant antitumor activity of T-DXd in G/GEJ cancer patients in the third or later line of treatment [42]. The currently recruiting DESTINY-gastric04 (DG-04) study (NCT04704934) investigates T-Dxd in patients with HER2-positive GC or GEJ adenocarcinoma who have progressed on or after a trastuzumab-containing regimen, and have not received any additional systemic therapy. The study design was presented at ESMO 2021 with OS as primary endpoint.
The key secondary endpoints include PFS, ORR, and immunogenicity of T-DXd. Patients are enrolled in 18 study locations in France, Japan, Korea and Singapore; estimated study completion date is November 2024 [43].
REFERENCES
- Rawla P et al., Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad gastroenterologiczny 2019; 14(1): 26-38
- Janjigian YY et al., Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal denocarcinoma (GC/GEJC/EAC): CheckMate 649 study. ESMO 2021, LBA7;
- Opdivo® (nivolumab) prescribing information
- Janjigian YY et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649):
a randomised, open-label, phase 3 trial. Lancet 2021; 398(10294): 27-40 - Chau I et al., Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol 39, 2021 (suppl; abstr LBA4001)
- Hoy SM, Sintilimab: First Global Approval. Drugs 2019; 79(3): 341-346
- Yang Y et al., Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020; 15(10): 1636-1646
- Wang J et al., Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs 2019; 11(8): 1443-1451
- Zhang L et al., Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol 2020; 10: 594558
- Xu J et al., Sintilimab plus chemotherapy (chemo) versus chemo as the first line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT 16). ESMO 2021, LBA53
- Deng J et al., Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nature communications 2017; 8(1): 1533-1533
- Shen L et al., Sintilimab plus chemotherapy versus chemotherapy as first line therapy in patients with advanced or metastatic esophageal squamous cell cancer: First Results of the Phase 3 ORIENT 15 study. ESMO 2021, LBA52
- XU RH et al., JUPITER-06: A randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment naive advanced or metastatic esophageal squamous cell carcinoma (ESCC). ESMO 2021, 1373MO
- Nishi T et al., The Present Status and Future of Barrett’s Esophageal Adenocarcinoma in Japan. Digestion 2019; 99(2): 185-190
- Wall JA et al., The anti-DKK1 antibody DKN-01 as an immunomodulatory combination partner for the treatment of cancer. Expert Opin Investig Drugs 2020; 29(7): 639-644
- Haas MS et al., mDKN-01, a Novel Anti-DKK1 mAb, Enhances Innate Immune Responses in the Tumor Microenvironment. Mol Cancer Res 2021; 19(4): 717-725
- Kagey MH et al., Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br J Pharmacol 2017; 174(24): 4637-4650
- Klempner S et al., DKN-01 in combination with tislelizumab and chemotherapy as a first-line therapy in unselected patients with advanced gastroesophageal adenocarcinoma (GEA): DisTinGuish trial. ESMO 2021, 1384P
- Abnet CC et al., Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018; 154(2): 360-373
- Kelly RJ et al., Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021; 384(13): 1191-1203
- Ilson DH Adjuvant Nivolumab in Esophageal Cancer – A New Standard of Care. N Engl J Med 2021; 384(13): 1269-1271
- Kang X et al., Adjuvant immunotherapy for resected esophageal squamous cell carcinoma with high risk of recurrence (AIRES): A multicenter, open-label, randomized, controlled phase III trial. ESMO 2021, 1435TiP
- El-Khoueiry AB et al., Phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology 2020; 38(4_suppl): 564-564
- Pavlakis N et al., Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016; 34(23): 2728-35
- Pavlakis N et al., INTEGRATE IIb: A randomised phase III open label study of regorafenib + nivolumab vs standard chemotherapy in refractory advanced gastro-oesophageal cancer (AGOC). ESMO 2021, 1438TiP
- Liu R et al., Phase I study of pucotenlimab (HX008), an anti-PD-1 antibody, for patients with advanced solid tumors. Ther Adv Med Oncol 2021; 13: 17588359211020528
- Zhang J et al., HX008: a humanized PD-1 blocking antibody with potent antitumor activity and superior pharmacologic properties. mAbs 2020; 12(1): 1724751-1724751
- Song Y et al., HX008, an anti-PD1 antibody, plus irinotecan as second-line treatment for advanced gastric or gastroesophageal junction cancer: a multicenter, single-arm phase II trial. Journal for immunotherapy of cancer 2020; 8(2): e001279
- Xu J et al., Anti-PD-1 antibody HX008 combined with oxaliplatin plus capecitabine for advanced gastric or esophagogastric junction cancer: a multicenter, single-arm, open-label, phase Ib trial. Oncoimmunology 2020; 10(1): 1864908-1864908
- Huang J et al., A randomized, double-blinded, placebo-controlled phase III trial of HX008 plus irinotecan as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma. ESMO 2021, 1439TiP
- Kam AE et al., Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol 2021; 6(11): 956-969
- Meric-Bernstam F et al., Presented at ASCO GI 2021. Abstract 164
- Ku G et al., Phasea (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). ESMO 2021, 1380P
- FDA approval margetuximab.
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-margetuximab-metastatic-her2-positive-breast-cancer, last accessed October 2021 - Catenacci DV et al., MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol 2021; 17(10): 1155-1164
- Catenacci DV et al., Margetuximab (M) with retifanlimab (R) in HER2+, PD-L1+ 1st-line unresectable/metastatic gastroesophageal adenocarcinoma (GEA): MAHOGANY cohort A. ESMO 2021, 1379P
- Jiang C et al., Engineering a Smart Agent for Enhanced Immunotherapy Effect by Simultaneously Blocking PD-L1 and CTLA-4. Adv Sci (Weinh) 2021: e2102500
- Catenacci DVT et al., Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol 2020; 21(8): 1066-1076
- Gong J et al., Preliminary Efficacy and Safety Results of KN026 (a HER2-targeted Bispecific Antibody) in Combination with KN046 (an anti-PD-L1/CTLA-4 Bispecific Antibody) in Patients (pts) with HER2-positive Gastrointestinal Tumors. ESMO 2021, 1377P
- Shitara K et al., Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020; 382(25): 2419-2430
- Zhao D et al., Progress and challenges in HER2-positive gastroesophageal adenocarcinoma. Journal of hematology & oncology 2019; 12(1): 50-50
- Yamaguchi K et al., Presented at ASCO Annual Meeting 2021; Poster 4048
- Shitara K et al., Trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-positive gastric cancer (GC) or gastroesophageal junction (GEJ) adenocarcinoma who have progressed on or after a trastuzumab-containing regimen (DESTINYgastric04, DG-04): A randomized phase III study. ESMO 2021, 1436TiP
© 2021 Springer-Verlag GmbH, Impressum
More posts
Highlights in cervical cancer
Highlights in cervical cancer In 2020, more than 600,000 new cases of cervical
Novel approaches in gastric/gastroesophageal cancer
Novel approaches in gastric/gastroesophageal cancer Gastric cancer (GC) is the
Colorectal cancer – personalized medicine for a heterogeneous disease
Colorectal cancer – personalized medicine for a heterogeneous disease Prof. Chia
New horizons in colorectal cancer
New horizons in colorectal cancer FOLFIRINOX: real-world data in first line tre
Preface
Preface – ESMO 2021 Yelena Y. Janjigian, MD Memorial Sloan Kettering Cance