Advances in immunotherapy for neuroendocrine tumors
Limited activity of checkpoint inhibitors as monotherapy
Chemotherapy is currently the SoC first-line treatment for high-grade neuroendocrine neoplasms (HG-NENs), even though it only provides modest benefits in OS and PFS [1]. Given the lack of therapeutic options for metastatic NEN patients and the promising antitumor activity of immunotherapy demonstrated across several solid cancer types, the efficacy of pembrolizumab monotherapy was investigated in an open-label, nonrandomized phase II study in patients with metastatic extra-pulmonary HG-NEN (Ki67 >20 %) [2].
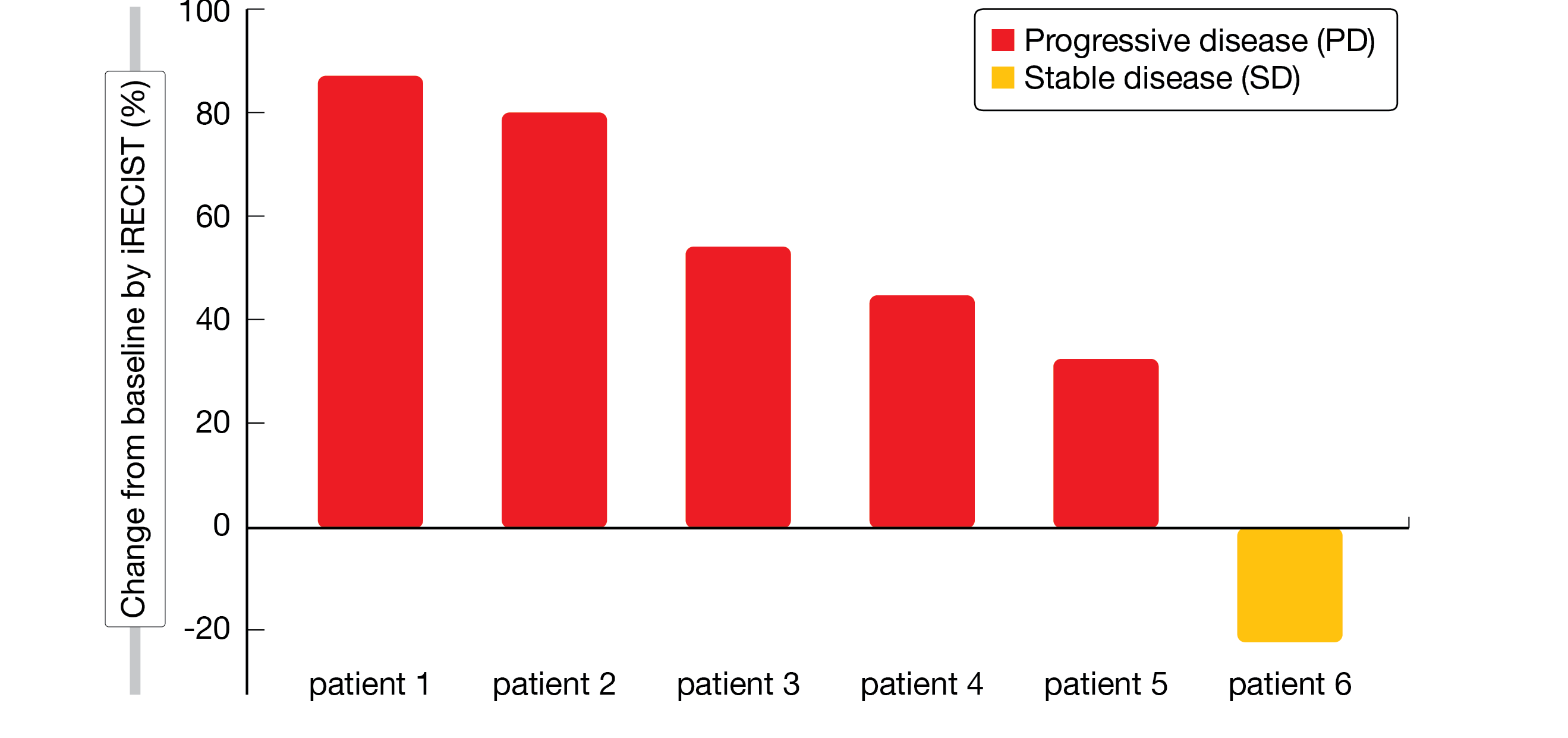
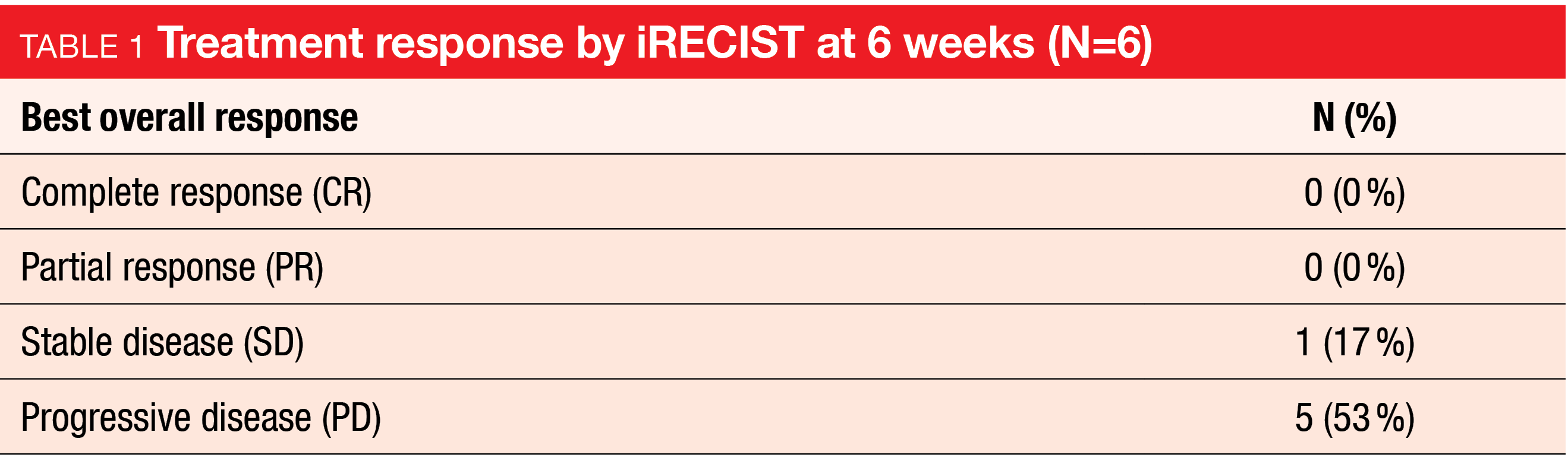
Six patients who had progressed upon platinum- or temozolomide-based chemotherapy were included in the study and received at least 1 dose of pembrolizumab. The authors reported that one patient had stable disease, which was maintained for 8.3 months, while the remaining 5 had progressive disease at 6 weeks (Figure 1, Table 1). The treatment was well tolerated and only one AE of grade ≥3 was considered to be related to the drug.
Despite the small number of patients, these results already indicated that pembrolizumab has limited activity as monotherapy in HG-NENs. These findings were consistent with previously published studies assessing pembrolizumab’s efficacy in metastatic grade 3 NENs [3, 4].
Figure 1: Percent change in target tumor size from baseline at 6 weeks of pembrolizumab monotherapy
Conflicting results of different combinations of ICIs with TKIs: tislelizumab plus surufatinib…
As outlined above, the efficacy of immune checkpoint inhibitors (ICIs) as monotherapy in NETs has been disappointing [2-4]. However, the combination with tyrosine kinase inhibitors (TKIs), which has proven effective in other cancers such as endometrial cancer or renal cell carcinoma [5, 6], remains to be further explored in NETs. The inhibition of angiogenesis together with the stimulation of an immune response may have a synergistic effect and enhance overall antitumor activity.
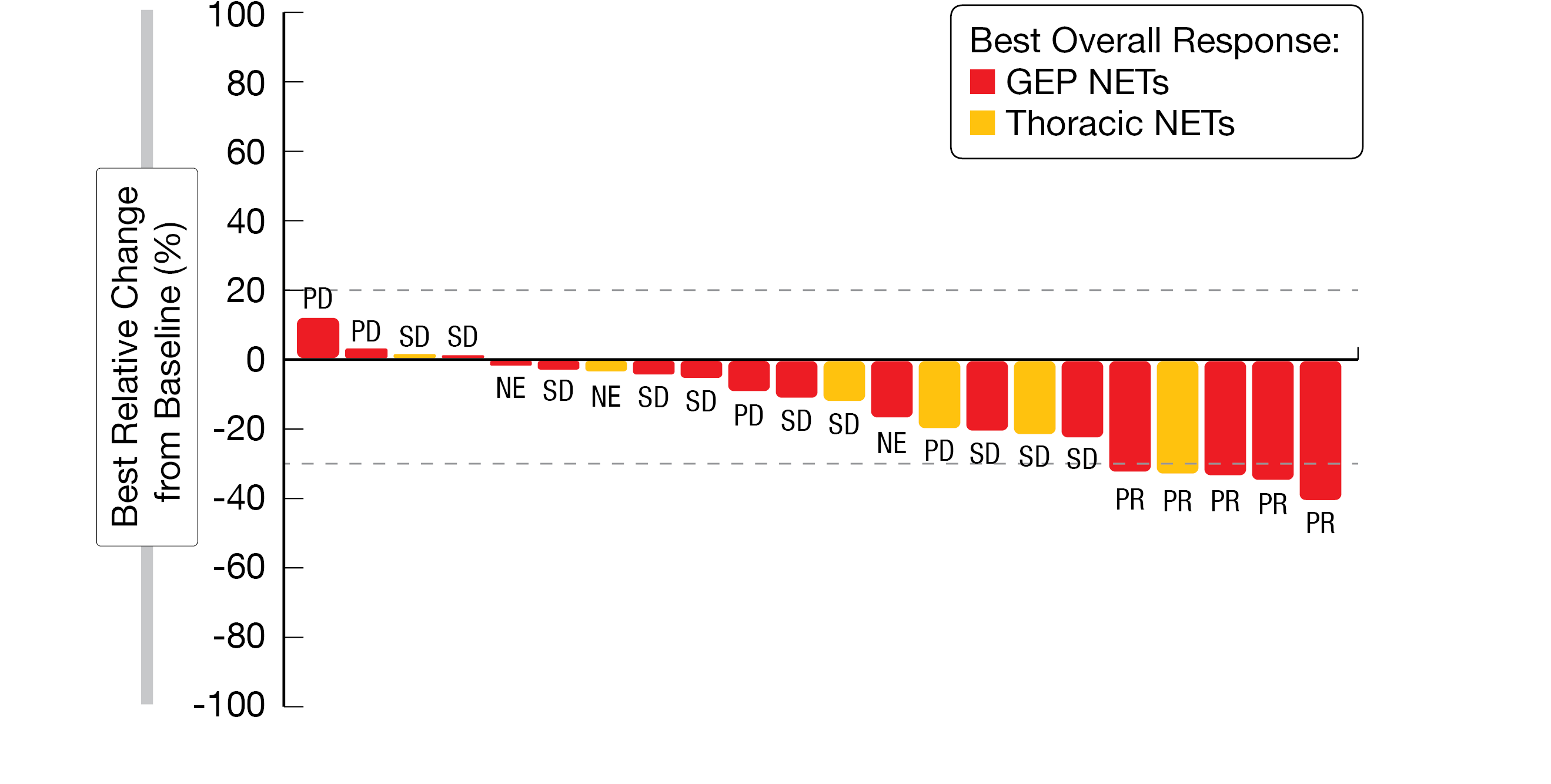
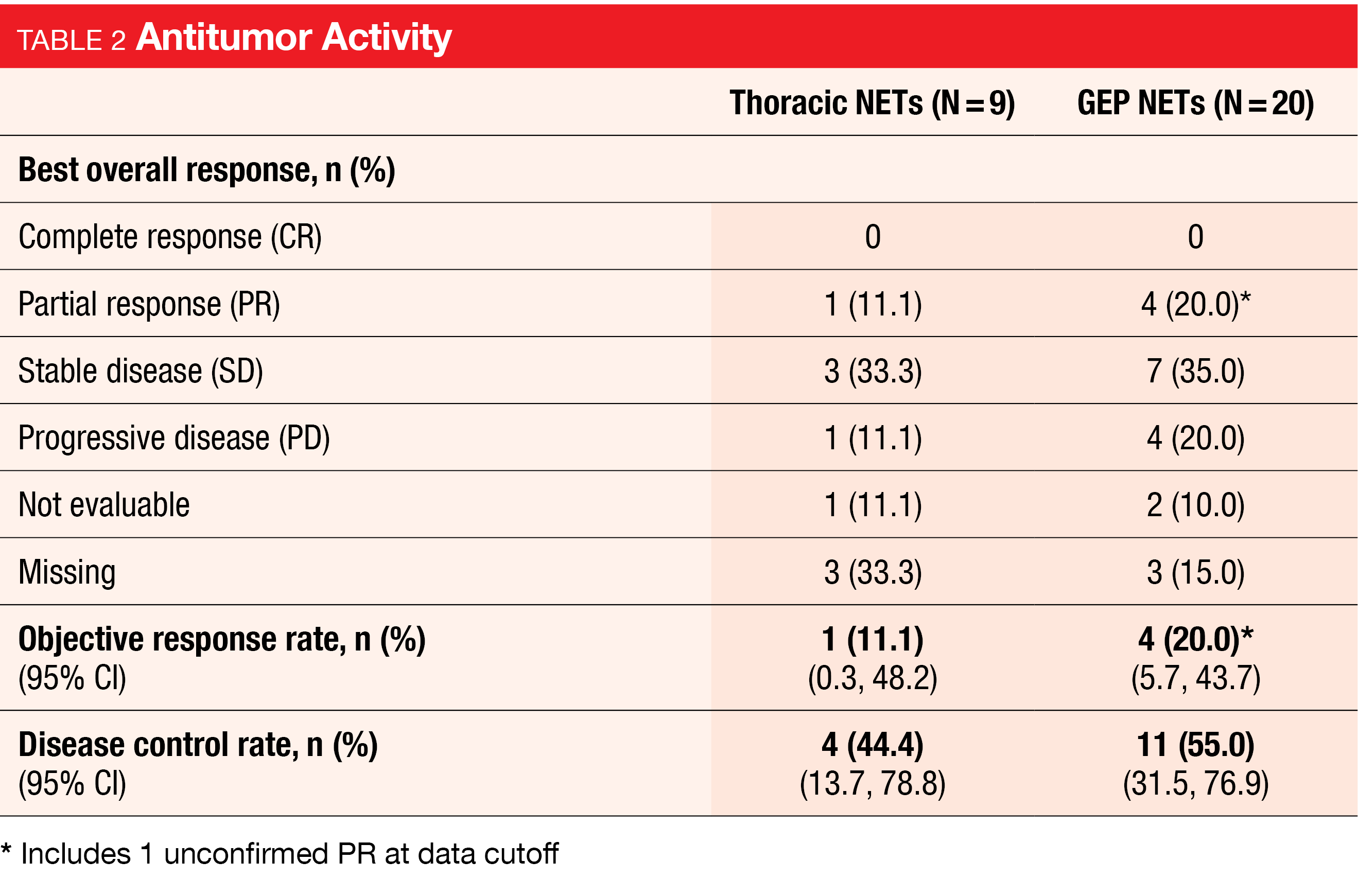
The open-label, phase 1b/2 dose escalation/expansion study presented by Eads et al. explored the preliminary antitumor activity of tislelizumab, an anti-PD-1 monoclonal antibody, plus surufatinib, a TKI, in thoracic- and GEP-NETs. Twenty-nine NET patients who had received prior anticancer treatment were enrolled in the expansion study, where they received 300 mg of surufatinib orally, once daily (RP2D established in escalation), and 200 mg of tislelizumab intravenously every 3 weeks. No patient demonstrated a complete response. However, partial responses were observed in five patients (17.2 %), and 10 patients had stable disease (34.5 %) (Figure 2). The reported ORR was 11.1 % for the thoracic NET cohort and 20 % for the GEP-NET cohort (Table 2). While at least one TEAE of any grade occurred in all 29 patients, TEAEs of grade ≥3 were noted in 20 patients (69 %). The most common TEAEs were increased aspartate aminotransferase (AST) (51.7 %), nausea and hypertension (44.8 % each), decreased appetite and fatigue (41.4 % each), and increased alanine aminotransferase (ALT) (34.5 %). One case each of increased AST and ALT led to dose reductions in the GEP-NET cohort [7].
The authors concluded that surufatinib plus tislelizumab demonstrated encouraging anti-tumor activity and manageable safety in pre-treated patients with NETs.
…and pembrolizumab plus lenvatinib
In contrast with the previous findings, the combination of pembrolizumab with the multitargeted TKI lenvatinib assessed in an open-label phase II trial presented at NANETS 2022 did not demonstrate sufficient response in patients with advanced gastrointestinal and thoracic NETs [8]. This prospective study included patients with well-differentiated NETs who had received at least two prior lines of systemic treatment and showed evidence of disease progression within 8 months of study entry. Study participants were administered 20 mg of lenvatinib orally daily and 200 mg of pembrolizumab intravenously every three weeks until unacceptable toxicity or progressive disease.
In an interim analysis of the first 20 patients enrolled in the study, only two reached a partial response (10 %). The median PFS was 9 months. Probably- or definitely-associated grade 3 AEs were reported by 12 patients (60 %), and 14 patients (70 %) required dose reductions or discontinued one of the treatments. Since not even 4 ORs were reached, further enrolment was not warranted.
Figure 2: Best percent change in target lesion diameter with tislelizumab plus surufatinib in thoracic and GEP NETs
Potential of oncolytic viruses to sensitize tumors to checkpoint inhibitors: early-phase trials and preclinical data
It has been proposed that the limited activity of ICIs in NENs may be due to a non-inflamed phenotype of their tumor microenvironment [9]. Based on this assumption, several studies started to explore the potential of oncolytic viruses to convert NENs to a highly inflamed phenotype, which would sensitize them to ICI therapy [10, 11].
An engineered Vesicular stomatitis virus-based oncolytic virus (VSV-IFNβ-NIS) is currently being tested in combination with pembrolizumab in a phase 1/2 trial in neuroendocrine carcinoma (NEC) patients who have progressed on at least one prior line of systemic therapy. As reported at NANETS 2022 by McGarrah et al., the safety run-in phase of the study has been completed and the enrolment of patients for the dose expansion cohort was ongoing at the time of presentation. Twelve NEC patients of any primary tumor site will be included in the study and treated with the RP2D of VSV-IFNβ-NIS on day 1, followed by pembrolizumab on day 8 and then every 3 weeks until progression of disease or unacceptable toxicity, for up to 2 years. The primary endpoint will be ORR by RECIST v1.1, and if at least one objective response is observed and safety is confirmed, the study regimen will be considered for further investigation [10].
The Seneca Valley virus (SVV), on the other hand, is a naturally occurring oncolytic virus found to have selectivity for NETs. In a preclinical study presented at NANETS 2022, the efficacy of SVV in combination with ICIs was evaluated using an ICI-resistant mouse model. SVV was intratumorally injected along with systemic ICIs (anti-PD-1 and/or anti-CTLA4). Complete responses were observed in 5 out of 6 (>83 %) tumor-bearing mice within 44 days of injection of SVV+anti-PD-1+anti-CTLA4, and these animals remained tumor-free for >160 days. In contrast, control-treated mice were all sacrificed by day 70 (median survival <50 days) due to tumor burden, with transient tumor regressions observed only in those treated with anti-PD-1 plus anti-CTLA4. In addition, tumors from mice injected with the combination of SVV+ICIs showed the highest levels of CD3+ and CD8+ T-cell infiltration, which indicates conversion to an inflamed phenotype. This study provided evidence that SVV is able to reverse resistance to ICIs and enhance their efficacy. Based on these promising preclinical data, a first-in-human phase I trial of SVV oncolytic virotherapy combined with ICIs is expected to start enrolling NEN patients in the first quarter of 2023 [11].
Novel immunotherapies in the NET setting: from CAR T-cells…
Adoptive cell therapy using chimeric antigen receptor (CAR) T-cells has proven remarkably effective in patients with B-cell malignancies, but there is a lack of data regarding solid tumors, including NETs [12]. Results from preclinical studies presented at NANETS 2022 showed that an anti-SSTR CAR construct, which was developed to direct T-cells against SSTR-positive NETs by incorporating the SSA octreotide in the extracellular domain, demonstrated promising cytotoxic activity both in vitro and in vivo [13]. The design of a clinical trial to evaluate its toxicity is currently under development. Since the potential for toxicities and side effects associated with CAR T-cells is very high, the authors underlined that even if no toxicities of the construct were observed in SSTR-expressing organs in mice, only patients who have progressed after previous lines of treatment and who have exhausted all other options will be included in the clinical trial.
… to a survivin-targeting vaccine…
SurVaxM is a novel immunotherapy based on a peptide vaccine that targets survivin, a cell-survival protein expressed in 95 % of glioblastomas and many other cancers, including NETs [14]. A subset of NET patients with survivin-expressing tumors could potentially benefit from a survivin-targeting therapy, as survivin expression has been shown to correlate with poorer outcomes in NETs (OS 8.5 years vs. 18.3 years) [15].
An ongoing phase I trial is currently assessing the safety and immunogenicity of SurVaxM in patients with survivin-positive (>1% by immunohistochemistry) metastatic NETs. With enrolment still ongoing at the time of presentation, 4 patients had completed the treatment (4 doses every 2 weeks), and the observed PFS reported at NANETS 2022 was 10.9 months [16].
… and a somatostatin-based bispecific T-cell engager
Another novel immunotherapy currently under development for NETs is based on bispecific antibodies, which are designed to target a specific tumor-associated antigen as well as to engage and activate tumor-infiltrating lymphocytes [17].
With the aim of targeting well-differentiated NETs, Pelle et al. investigated a hormone-based bispecific T-cell engager (BiTE) composed of two molecules of somatostatin-14 linked with a single-chain variable fragment-based anti-CD3. In a preclinical setting, this BiTE-like molecule was shown to specifically engage the T-cell receptor CD3 (>85 % of T-cells bound at a concentration of 100 nm) and to efficiently induce a high level of SSTR-specific T-cell activation, as indicated by the significantly increased secretion of IFN-γ detected in the presence of SSTR-expressing cells (p<0.0001) [18]. Based on these promising results, further studies will be aimed at determining the efficacy of the BiTE in vivo.
REFERENCES
- Alheraki SZ et al., Treatment landscape of advanced high-grade neuroendocrine neoplasms. Clin Adv Hematol Oncol 2023; 21(1): 16-26
- Hunter LA et al., Pembrolizumab for the treatment of recurrent high grade neuroendocrine neoplasms. NANETS 2022, abstract C-2
- Vijayvergia N et al., Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms:
joint analysis of two prospective, non-randomised trials. Br J Cancer 2020; 122(9): 1309-1314 - Strosberg J et al., Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the Phase II KEYNOTE-158 study. Clin Cancer Res 2020; 26(9): 2124-2130
- Makker V et al., Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 2022; 386(5): 437-448
- Motzer R et al., Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384: 1289-1300
- Eads JR et al., An open-label, Phase 1b/2 study of surufatinib in combination with tislelizumab in patients with advanced neuroendocrine tumors. NANETS 2022, abstract C-6
- Al-Toubah T et al., Phase II study of pembrolizumab and lenvatinib in advanced well-differentiated neuroendocrine tumors. NANETS 2022, abstract C-8
- Takkenkamp TJ et al., The immune tumour microenvironment of neuroendocrine tumours and its implications for immune checkpoint inhibitors. Endocr Relat Cancer 2020; 27(9): R329-R343
- McGarrah PW et al., Phase 1-2 trial of Vesicular stomatitis virus expressing human Interferon-β and NIS (VSVIFNβ-NIS), with pembrolizumab, in patients with neuroendocrine carcinoma. NANETS 2022, abstract T-4
- Hallenbeck PL et al., Oncolytic Seneca Valley virus (SVV-001) overcomes checkpoint inhibitor resistance and demonstrates a systemic anti-tumor response in a syngeneic tumor model. NANETS 2022, abstract B-5
- Jackson HJ et al., Driving CAR T-cells forward. Nat Rev Clin Oncol 2016; 13(6): 370-83
- Mandriani B et al., Development of anti-somatostatin receptors CAR T cells for treatment of neuroendocrine tumors. J Immunother Cancer 2022; 10(6): e004854
- Ahluwalia MS et al., Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J Clin Oncol 2022; JCO2200996
- Hanif A et al., Exploring the role of survivin in neuroendocrine neoplasms. Oncotarget 2020; 11(23): 2246-2258
- Iyer R, Development of survivin vaccine for NETs. NANETS 2022, oral presentation (no abstract).
- Thakur A et al., Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev 2018; 32(4): 339-347
- Pelle E et al., Development of a novel anti-SSTR bispecific T-cell engager (BiTE)-like molecule for the treatment of neuroendocrine tumors. NANETS 2022, abstract B-3
© 2023 Springer-Verlag GmbH, Impressum
More posts
Advances in immunotherapy for neuroendocrine tumors
Advances in immunotherapy for neuroendocrine tumors Limited activity of checkpo
SSTR-positive neuroendocrine tumors: peptide receptor radionuclide therapy
SSTR-positive neuroendocrine tumors: peptide receptor radionuclide therapy Real
Preface – NANETS 2022
Preface – NANETS 2022 © private - Mauro Cives, MD, Department of Interdisciplinary Me