Notable advances in the field of anti-EGFR therapy
The irreversible ErbB family blocker afatinib and the reversible EGFR TKIs gefitinib and erlotinib have been approved as first-line therapies for treatment of NSCLC patients with EGFR-sensitising mutations. However, resistance frequently develops, which indicates the need for new agents. The EGFR T790M mutation has been identified as the most common resistance mutation.
The oral, irreversible, third-generation EGFR TKI osimertinib is active in both sensitising and EGFR T790M resistance mutations. This treatment was evaluated in AURA3, the first randomised phase III trial to compare a T790M-selective EGFR TKI with platinum-based doublet chemotherapy in patients with T790M-positive advanced NSCLC progressing on first-line EGFR TKI therapy [1]. Osimertinib was administered at 80 mg once daily (OD) in the experimental arm (n = 279), while patients in the control arm received pemetrexed plus carboplatin or cisplatin, followed by optional pemetrexed maintenance (n = 140). Stable asymptomatic central nervous system (CNS) metastases were allowed.
AURA3: 70 % risk reduction with osimertinib
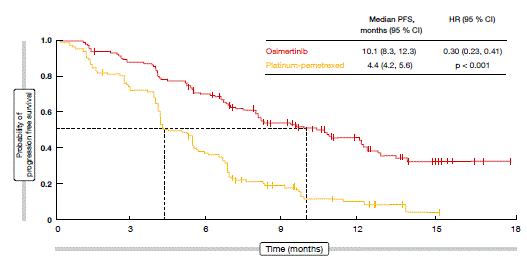
Osimertinib demonstrated statistically superior and clinically meaningful activity compared to the platinum-pemetrexed therapy. The primary endpoint of investigator-assessed PFS was highly significantly in favour of osimertinib (10.1 vs. 4.4 months; HR, 0.30; p < 0.001; Figure 1). Progression-free survival (PFS) benefits occurred across all of the subgroups. Patients with CNS metastases at baseline experienced similar reductions in the risk of progression or death (PFS, 8.5 vs. 4.2 months; HR, 0.32) as those without cerebral lesions (10.8 vs. 5.6 months; HR, 0.40). The objective response rate (ORR) was significantly higher with osimertinib (71 % vs. 31 %; p < 0.001), and the median duration of response was longer (9.7 vs. 4.1 months). Moreover, the tolerability of osimertinib surpassed that of chemotherapy, as possibly treatment-related grade ≥3 adverse events (AEs) occurred less frequently (6 % vs. 34 %). The investigators thus noted that osimertinib represents the new standard of care for patients with EGFR T790M-positive NSCLC following disease progression with first-line EGFR TKI therapy.
Figure 1: PFS according to investigator assessment in AURA3: pronounced advantage for osimertinib over chemotherapy
According to another analysis of AURA3, the clinical benefits obtained with osimertinib in this trial were independent of whether T790M positivity had been established by testing of tissue or for circulating tumour DNA (ctDNA) [2]. Sensitivity and specificity rates for T790M detection in the plasma using the cobas® EGFR Mutation Test v2 as a reference were 51 % and 77 %, respectively. The analysis revealed high sensitivity and specificity for both exon 19 deletion and L858R mutation. PFS and ORR were similar for T790M-positive patients according to tumour tissue and ctDNA testing. This is a favourable finding, as re-biopsy at disease progression is not always feasible, and can be associated with risks and treatment delays.
LUX-Lung 7: continued benefit with afatinib over gefitinib
The phase IIB LUX-Lung 7 trial was the first prospective, global, randomised study to compare two EGFR-directed therapies (afatinib and gefitinib) head-to-head in the first-line setting. A total of 319 patients with EGFR-positive stage IIIB/IV adenocarcinoma of the lung were randomised to either afatinib 40 mg OD or gefitinib 250 mg OD. In the primary analysis, afatinib significantly improved the co-primary endpoints of PFS and time to treatment failure (TTF) compared to gefitinib [3]. The key secondary endpoint, ORR, was also significantly improved. At the WCLC, Park et al. presented the primary overall survival (OS) analysis as well as other updated outcomes [4].
The OS did not differ significantly between these two arms, although a 14 % reduction in the risk of death occurred for afatinib (median OS, 27.9 vs. 24.5 months, for afatinib vs. gefitinib; HR, 0.86; p = 0.2580). The trend favouring afatinib was consistent across pre-specified subgroups, including populations with deletion 19 (30.7 vs. 26.4 months; HR, 0.83) and L858R mutation (25.0 vs. 21.2 months; HR, 0.91). Independently reviewed PFS still showed benefit with afatinib treatment (11.0 vs. 10.9; HR, 0.74; p = 0.0178), as did the updates for TTF (13.7 vs. 11.5 months; HR, 0.75; p = 0.0136) and ORR (73 % vs. 56 %; OR, 2.12; p = 0.002). Median duration of response was 10.1 vs. 8.3 months.
The updated quality-of-life data were also similar between these arms. AEs were predictable and manageable, with equally low rates of treatment discontinuation. Dose reductions of afatinib improved toxicity without compromising efficacy. Patients who received dose reductions within the first 6 months of treatment experienced similar median PFS results as those who were treated with afatinib ≥ 40 mg OD for the first 6 months (12.8 and 11.0 months, respectively).
Findings in elderly patients
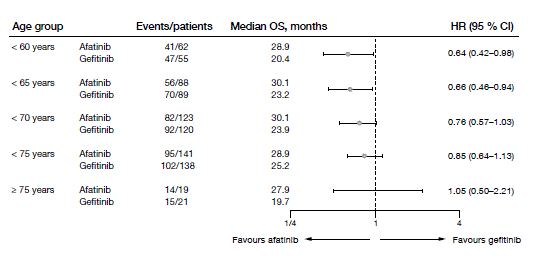
As more than one third of patients with lung cancer are at least 75 years old, the efficacy and safety of new agents matters in this population. Treatment can be challenging due to poorer functional status and high comorbidity burden. According to post-hoc subgroup analyses of patients aged ≥ 75 and < 75 years in LUX-Lung 7, advanced age did not adversely affect the outcomes achieved with afatinib versus gefitinib [5]. PFS and OS findings were consistent across age subgroups (Figure 2).
Figure 2: Median OS obtained with afatinib vs. gefitinib in various age groups in the LUX-Lung 7 trial
Afatinib demonstrated a predictable and manageable safety profile. In patients aged ≥ 75 years, no new or unexpected AEs emerged. These results suggest that afatinib can provide effective and tolerable treatment for older patients with EGFR-mutant NSCLC.
Predictors of long-term response in LUX-Lung 8
The randomised, open-label phase III LUX-Lung 8 study compared afatinib 40 mg OD and erlotinib 150 mg OD in patients with squamous-cell carcinoma (SCC) of the lung who had progressed after ≥ 4 cycles of platinum-doublet chemotherapy. Here, afatinib significantly improved PFS and OS (HR, 0.81 for both) [6], which prompted its approval for this indication. A group of 15 long-term responders (LTRs) who derived prolonged benefit from afatinib treatment was identified in the LUX-Lung 8 trial. In this cohort, the median treatment duration was 16.6 months. Goss et al. investigated molecular and clinical biomarkers that might be indicative of long-term response to afatinib [7].
The baseline characteristics of the LTRs did not deviate to any meaningful extent from those of the overall afatinib-treated population. Also, the best responses to first-line chemotherapy were similar across these two groups. Median OS and PFS in the LTRs were 23.1 months and 16.2 months, respectively. One patient experienced CR, four patients had PR, and eight patients had SD. Next-generation sequencing was performed for nine of the LTRs and for 132 of the 398 afatinib-treated patients in the overall study population. This analysis showed that certain short variants were more common in the LTRs, such as aberrations in the ErbB family, MLL, KEAP1 and PIK3CA genes. Copy number aberrations occurred with similar incidence across these two groups. According to the VeriStrat® proteomic assay, a greater proportion of the LTRs was classified as “Good” compared to the overall afatinib-treated population (86 % vs. 62 %). These patients were nearly four times as likely to survive for ≥ 12 months compared to the “VeriStrat®-Poor” patients.
The frequency of common treatment-related AEs in the LTRs was similar to that observed in the overall afatinib-treated population. Afatinib 40 mg OD was maintained in seven of the 15 LTRs, with escalation to afatinib 50 mg in four. Dose reductions did not appear to affect OS adversely. Further studies are required to predict longterm responses to afatinib in patients with SCC of the lung.
In the overall patient population of LUX-Lung 8, however, Felip et al. identified no tumour biomarkers that affected outcome [8]. Although the samples of these patients included multiple genetic aberrations, no biomarkers were predictive of clinical outcomes with afatinib or erlotinib. PFS and OS did not differ significantly between afatinib and erlotinib in the “VeriStrat®-Poor” group. The investigators thus concluded that afatinib is more effective than erlotinib and should be considered as a second-line option in patients with SCC of the lung, regardless of tumour characteristics.
CSF penetration of afatinib
The CNS is a common site for tumour recurrence, probably due to the low penetration of some therapeutic agents through the blood-brain barrier. Patients with brain metastases arising from NSCLC have poor prognosis. Results from the LUX-Lung 3 and 6 studies suggest that afatinib is effective for the treatment of EGFR-positive NSCLC patients with brain metastases [9].
Tamiya et al. therefore prospectively analysed the cerebrospinal fluid (CSF) penetration rate of afatinib in 11 patients with EGFR-positive NSCLC and leptomeningeal carcinomatosis [10]. They showed that the median CSF penetration rate of afatinib of 1.7 % was higher than previously reported (0.7 %) [11]. The efficacy of afatinib in leptomeningeal carcinomatosis was demonstrated in particular for patients with uncommon EGFR mutations, such as exon 18 mutation. With regard to the toxicity, stomatitis, diarrhoea and skin complications required special attention.
Afatinib in medically unfit patients
As the LUX-Lung 3 and 6 trials solely included patients suitable for platinum-based doublet chemotherapy, the efficacy and toxicity of afatinib in patients not eligible for this kind of treatment remained unknown. One study suggested that TKIs can benefit medically unfit EGFR-mutant East Asian patients [12]. The single-arm, phase II TIMELY trial was the first on this issue to be conducted in a western population [13]. Thirty-nine patients with NSCLC who were deemed unsuitable for radical treatment or chemotherapy, or who declined the latter, participated in the study. They had either confirmed activating EGFR mutation or showed clinical characteristics that were indicative of EGFR mutations when no tissue was suitable for genotyping, or genotyping had failed/ was not available. Treatment consisted of afatinib 40 mg OD until progression.
At 6 months, 58 % of all patients were alive and progression-free (primary endpoint). Median PFS and OS were 7.9 and 15.5 months, respectively. In patients with confirmed EGFR mutation, PFS and OS were 10.2 months and had not been reached, respectively. Those with suspected EGFR mutants fared a bit worse in comparison (4.4 and 10.9 months, respectively), although these PFS and OS results appeared improved compared to similar patients who were considered unfit for chemotherapy in the TOPICAL trial [14]. The toxicity rate observed in TIMELY was higher than that usually seen in fitter patients. Twenty-three of the 39 patients experienced at least one grade ≥ 3 toxicity.
Icotinib is superior to brain irradiation
Whole-brain irradiation (WBI) has been a standard of care for NSCLC patients with brain metastases. The randomised phase III BRAIN trial evaluated the EGFR TKI icotinib at 125 mg three times daily compared to WBI with or without chemotherapy in EGFR-TKI–naïve patients with EGFR-mutant, advanced NSCLC and brain metastases at ≥ 3 sites [15]. In both arms, more than 80 % of the patients did not experience any symptoms related to their cranial lesions. Eighty-five and 73 patients received icotinib and WBI, respectively. Intracranial PFS was defined as the primary endpoint. BRAIN represents the first phase III trial to compare an EGFR TKI with WBI.
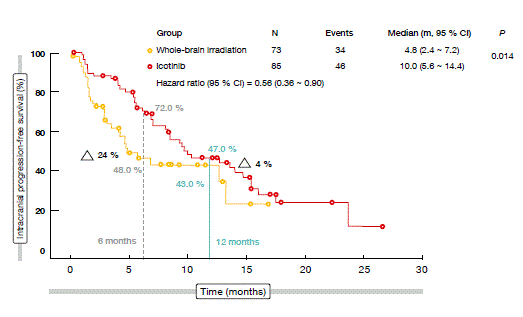
According to this analysis, icotinib significantly improved intracranial PFS over WBI (median, 10.0 vs. 4.8 months; HR, 0.56; p = 0.014). At 6 months, there was a 24 % difference in favour of icotinib (72.0 % vs. 48.0 %; Figure 3).
Figure 3: Intracranial PFS with icotinib vs. whole-brain irradiation ± chemotherapy
A significant benefit was also observed for PFS (6.8 vs. 3.4 months; HR, 0.44; p < 0.001). Six-month PFS rates achieved with icotinib and WBI were 55.0 % and 22.0 %, while at 1 year, 19.0 % versus 9.0 % of patients were alive and progression free. The OS analysis did not reveal any difference between the two arms. The icotinib treatment gave rise to significant benefits regarding intracranial ORR (67.1 % vs. 40.9 %; p < 0.001) and intracranial DCR (84.7 % vs. 67.1 %; p = 0.014). This was also true for overall ORR (55.0 % vs. 11.1 %; p < 0.001) and overall DCR (78.8 % vs. 54.8 %; p = 0.001). With respect to treatment-related toxicity, patients in the icotinib arm did better than the control group, with significant differences in favour of the EGFR TKI noted for AEs of all grades. Based on these data, the authors concluded that icotinib should be used in first-line treatment of advanced EGFR-mutant NSCLC patients with brain metastases.
Clinical significance of p53 mutation
Griesinger et al. reported the first data obtained in a homogeneously TKI-treated patient population with EGFR-activating mutations, to show that when classified as pathogenic versus nonpathogenic/ wild-type, p53 mutation is a negative predictive marker for PFS and OS [16]. Usually, p53 mutations are classified as either disruptive or nondisruptive. Here, the DNA-contact mutations R273C, R273G and R248Q were reclassified as pathogenic, as were missense mutations located inside loops L1-L3 of p53, along with sequence substitutions that reached a score of C65 according to the missense analysis programme Align-GVGD. All other p53 mutations located outside loops L1-L3 were scored as non-pathogenic.
According to the OS and PFS analyses, the impact of the p53 mutations was significant. In those with non-pathogenic/ wild-type mutations, median OS was 42 months, while those with pathogenic mutations had an OS of 23 months. For PFS, this was 18 and 11 months, respectively. As is known, patients with exon 19 mutation have a better prognosis than those with exon 21 mutation, but the prognostic and predictive impact of the p53 mutation held true for both of these groups. Also, p53 mutations were demonstrated to be a negative predictive factor irrespective of patient clinical characteristics (e.g., ECOG performance status, CNS metastases, smoking status). The investigators noted that patients with p53-mutated tumours who receive EGFR TKIs might require different therapy management. There is a need for further therapeutic approaches in this patient group, such as combinations of EGFR TKIs with other drugs.
Another analysis found that apart from the major resistance mutation T790M, the minor mutations L792F and C797S can develop in afatinib-resistant cells [17]. L792F and C797S appear to be sensitive to dacomitinib and erlotinib, respectively. To enable treatment with these agents, the authors recommended testing for these minor mutations in clinical practice when resistance to afatinib occurs.
REFERENCES
- Papadimitrakopoulou VA et al., Randomised phase III study of osimertinib vs platinum-pemetrexed for EGFR T790-positive advanced NSCLC (AURA3). WCLC 2016, PL03.03
- Wu YL et al., Osimertinib vs platinum-pemetrexed for T790M-mutation positive advanced NSCLC (AURA3): plasma ctDNA analysis. WCLC 2016, MA08.03
- Park K et al., Afatinib versus gefitinib as firstline treatment of patients with EGFR mutationpositive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577-589
- Park K et al., First-line afatinib versus gefitinib in EGFRm+ advanced NSCLC: updated overall survival analysis of LUX-Lung 7. WCLC 2016, OA23.05
- Park K et al., Afatinib versus gefitinib as firstline treatment for EGFR mutation-positive NSCLC patients aged ≥ 75 years: subgroup analysis of LUX-Lung 7. WCLC 2016, P3.02b-044
- Soria J-C et al., Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897-907
- Goss G et al., Second-line afatinib for advanced squamous cell carcinoma of the lung: analysis of afatinib long-term responders in the phase III LUX-Lung 8 trial. WCLC 2016, OA23.03
- Felip E et al., Second-line afatinib versus erlotinib for patients with squamous cell carcinoma of the lung (LUX-Lung 8): analysis of tumour and serum biomarkers. WCLC 2016, P3.02b-003
- Schuler M et al., First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor
gene mutations and brain metastases. J Thorac Oncol 2016; 11: 380-390 - Tamiya A et al., Efficacy and cerebrospinal fluid concentration of afatinib in NSCLC patients with EGFR mutation developing leptomeningeal
carcinomatosis. WCLC 2016, OA08.05 - Hoffknecht P et al., Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015; 10(1):156-63
- Inoue A et al., First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations
without indication for chemotherapy. J Clin Oncol 2009; 27(9): 1394-1400 - Popat S et al., Afatinib benefits patients with confirmed/suspected EGFR mutant NSCLC, unsuitable for chemotherapy (TIMELY phase II trial).
WCLC, P3.02b-046 - Lee SM et al., First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a doubleblind,
placebo-controlled, phase 3 trial. Lancet Oncol 2012; 13(11): 1161-1170 - Wu YL et al., BRAIN: a phase III trial comparing WBI and chemotherapy with icotinib in NSCLC with brain metastases harbouring EGFR mutations (CTONG 1201). WCLC 2016, PL03.05
- Griesinger F et al., TP53 mutations in EGFR mt+ NSCLC: a new predictive marker. WCLC 2016, MA04.05
- Kobayashi Y et al., EGFR T790M, L792F, and C797S mutations as mechanisms of acquired resistance to afatinib. WCLC 2016, P3.02b-120
More posts
Practice-changing refinements of lung cancer staging
Practice-changing refinements of lung cancer staging The 8th edition of the TNM
Anti-angiogenesis with nintedanib: activity in mesothelioma, and potential biomarkers
Anti-angiogenesis with nintedanib: activity in mesothelioma, and potential biomarke
Who is a candidate for immunotherapy?
Who is a candidate for immunotherapy? Johan Vansteenkiste, MD, PhD, Respiratory
Immunotherapy: novel anti-PD-L1 antibodies & various combination regimens
Immunotherapy: novel anti-PD-L1 antibodies & various combination regimens O
Liquid biopsy in the context of EGFR and other mutations
Liquid biopsy in the context of EGFR and other mutations Compared to tissue bio
Emerging treatments in ALK-positive NSCLC: new options, but also new challenges
Emerging treatments in ALK-positive NSCLC: new options, but also new challenges