Multiple approaches for the treatment and prevention of CNS metastases
Activity of afatinib and osimertinib in the EGFR-mutant setting
More than 40 % of NSCLC patients develop CNS metastasis in their lifetime [1, 2]. As the burden of brain lesions affects both quality of life and survival, the development of therapies that are able to penetrate the blood-brain barrier is an important focus of research.
Among the EGFR TKIs, the second-generation agent afatinib and the third-generation drug osimertinib have shown particular CNS activity in patients with EGFR-mutant NSCLC. Additional evidence on afatinib in this respect is provided by the analysis of a large, open-label, single-arm phase IIIb study conducted in EGFR TKI-naïve Asian patients in conditions similar to real-world clinical practice [3]. Among 479 patients, 92 had CNS lesions. PFS was numerically shorter in those with brain metastases compared to those without (10.9 vs. 12.4 months), but median time to symptomatic progression did not differ across the groups (14.8 vs. 15.4 months). In accordance with previous observations, the analysis of the total cohort confirmed that the use of tolerability-guided dose adjustments reduces the rates of commonly occurring AEs, while therapeutic efficacy of afatinib was maintained.
Kang et al. reported data on the activity of osimertinib in patients with brain lesions included in the Korean subset of the open-label, single-arm, real-world treatment ASTRIS trial [4]. ASTRIS investigated osimertinib 80 mg daily in a global population of patients with T790M-positive advanced NSCLC after previous EGFR TKI treatment. Patients with asymptomatic, stable CNS metastases who did not require increasing doses of corticosteroids within 2 weeks prior to initiation of osimertinib were allowed to enroll. This applied to 211 individuals.
The findings strongly supported the clinical benefits of osimertinib in patients with EGFR-mutant NSCLC and CNS metastases. Median PFS was 10.8 and 11 months, respectively, with 1-year PFS rates of 39.6 % vs. 47.3 %. Sixty-eight percent and 79.6 % of patients responded, and time to treatment discontinuation was 11.2 vs. 14.7 months.
ALK-positive pre-treated NSCLC: lorlatinib
The selective, potent ALK/ROS1 TKI lorlatinib was designed to penetrate the blood-brain barrier. In a phase I/II study, lorlatinib showed robust clinical activity in patients with ALK-positive NSCLC most of whom had CNS disease and had failed ≥ 1 ALK TKI [5, 6]. Cerebrospinal fluid sampling revealed a mean lorlatinib CSF-to-unbound plasma concentration ratio of 0.73, indicating high CNS penetration of the drug. Bauer et al. analysed the patients for progressive disease (PD), which was categorised as either CNS or non-CNS progression based on independent central review, or death [7]. The cumulative incidence rates were calculated using competing risks methodology in pooled cohorts in the ongoing phase II study that contains multiple subsets.
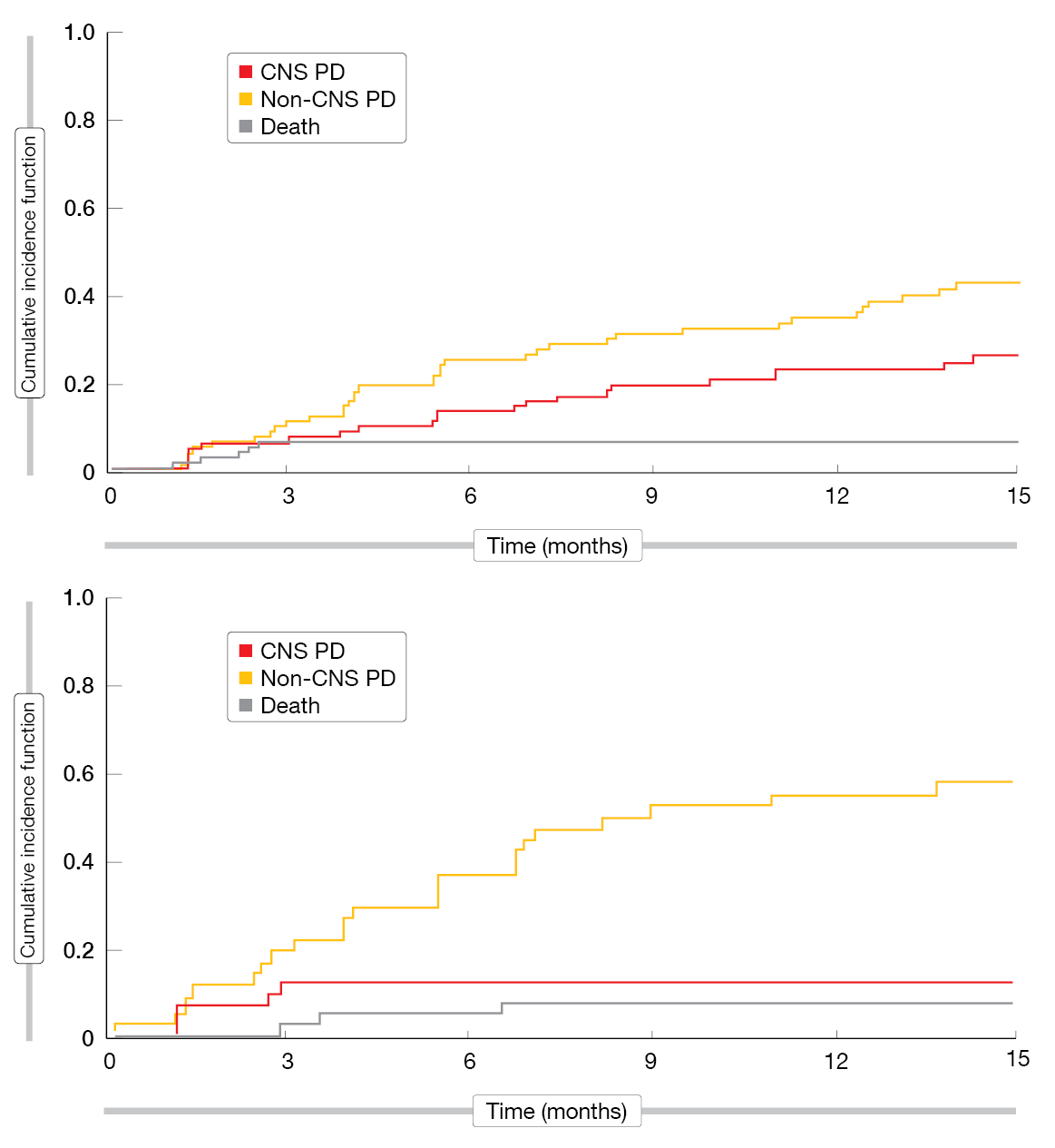
The analysis demonstrated pronounced activity of lorlatinib 100 mg daily in the treatment of brain lesions in patients with and without baseline CSN metastases after progression on crizotinib and/or second-generation ALK TKIs. In the group of crizotinib-pretreated patients, the intracranial ORR (IC-ORR) was 70 %, and median duration of intracranial responses (IC-DOR) had not been reached yet. For patients with baseline CNS metastases, the probabilities of both CNS and non-CNS PD were 22 % at 12 months. Those without brain lesions had a higher probability of non-CNS PD than CNS PD (43 % vs. 9 % at 12 months). Similarly, the patient group that had received one prior non-crizotinib TKI had an IC-ORR of 46 %, and median IC-DOR had not been reached. In those with two or three prior TKIs, IC-ORR and IC-DOR were 48 % and 15 months, respectively. Pooled data of those after a non-crizotinib TKI and those after two or three TKIs revealed that both patients with and without baseline CNS lesions showed a higher likelihood of extracranial PD compared to CNS PD (35 % vs. 23 % at 12 months for patients with baseline CNS metastases, and 55 % vs. 12 % for those without; Figure). This was also true for patients who had been treated with a second-generation ALK TKI as their last prior TKI therapy. Taken together, these findings underscore the activity of lorlatinib against CNS metastases and suggest that lorlatinib might also prevent the spread of the disease to the brain. To date, these are the only available prospective data on sequencing after progression on second-generation ALK TKI therapy.
Figure: Cumulative incidence of CNS progression, non-CNS progression, and death after ≥ 1 prior second-generation ALK TKI in lorlatinib-treated patients with (above) and without (below) baseline CNS metastases
Prophylactic cranial radiotherapy in high-risk patients
The role of prophylactic cranial irradiation (PCI) in patients with NSCLC remains controversial because of concerns about radiation-induced neurological morbidity and lack of OS gain. Arrieta et al. presented results showing that PCI is beneficial in patients with a high risk of developing brain metastases [8]. These were defined as patients showing a target mutation (e.g. either sensitising EGFR mutations or ALK rearrangement) and/or elevated CEA levels (> 20 pg/ml) at the time of diagnosis. In addition to treatment with first- and second-generation TKIs, they were randomised to receive either PCI (25 Gy in 10 fractions, 5 days per week; n = 41) or were followed up only (n = 43). Intracranial PFS constituted the primary outcome. After an amendment, patients who were treated with PCI after January 2016 had hippocampal sparing.
According to the multivariate analysis, PCI reduced the risk of intracranial progression and death by 60 % (p = 0.006). Patients treated with PCI showed a 22 % cumulative incidence of CNS progression at 24 months, while those in the control arm experienced CNS progression in 52 %. Similar trajectories were observed for OS (median OS, 42.8 vs. 25.9 months; HR, 0.47; p = 0.035). Cognitive function was assessed using Mini Mental State Examination (MMSE), and quality of life was evaluated through the EORTC-QLQ-30 questionnaire [9]. MMSE scores and median score values for global quality of life, fatigue and cognitive functioning did not differ across groups or between baseline and follow-up. Long-term assessments are necessary, however.
Overall, these results highlight the benefit of PCI particularly in patients at high risk of developing brain metastases. The authors noted that these findings can be extrapolated for patients treated with third-generation TKIs, which have higher CNS penetration but are often not accessible in developing countries.
Does immunotherapy work in the brain?
The multicentre, non-interventional, retrospective cohort IMMUNOBrainZH study was designed to evaluate the PD-1 inhibitor nivolumab 3 mg/kg Q2W in patients with advanced NSCLC and brain metastases who had failed ≥ 1 line of chemotherapy [10]. Fifty out of 77 eligible patients had received either stereotactic radiotherapy (SRT, n = 17) or whole-brain radiotherapy (WBRT, n = 33), while in 27 cases, no previous intracranial local treatment had been administered. PD-L1 expression levels were unknown.
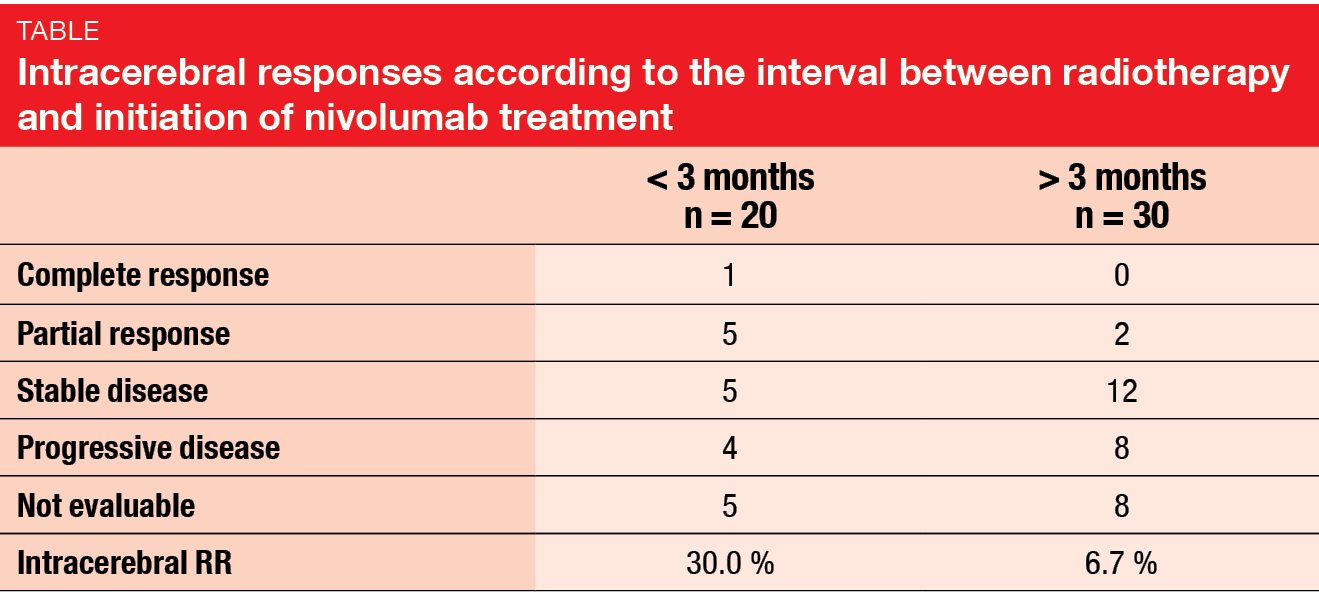
For intracerebral response, which was defined as the primary endpoint, the analysis yielded a rate of 20.8 %. Extracerebral responses occurred in 22.1 %, and the ORR was 23.4 %. When analysed according to prior local treatment, intracerebral response rates were higher in patients without previous radiotherapy (29.6 %) and those after SRT (23.5 %) than in those after WBRT (12.1 %). Patients who had received radiotherapy less than 3 months before nivolumab initiation responded considerably better than those with a longer interval (intracerebral RRs, 30.0 % vs. 6.7 %; Table). Intracerebral PFS was 8.0 months for the entire cohort, and OS was 9.0 months.
The authors concluded that intra- and extracerebral efficacy of nivolumab appears to be similar. Prior radiotherapy within 3 months of the beginning of nivolumab therapy might have a synergistic anti-tumour effect. Immunotherapy, as other systemic therapies, demonstrates promising efficacy on brain metastases due to NSCLC.
REFERENCES
- Oxnard GR et al., Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011; 17: 1616-1622
- Yu HA et al, Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19(8): 2240-2247
- Wu YL et al., A phase IIIb trial of afatinib in EGFRm+ NSCLC: analysis of outcomes in patients with brain metastases or dose reductions. WCLC 2018, P1.01-98
- Kang JH et al., Real world data of osimertinib in patients with central nervous system (CNS) metastasis in ASTRIS Korean subgroup. WCLC 2018, MA08.07
- Shaw AT et al., Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017; 18: 1590-1599
- Solomon B et al., Lancet Oncol 2018, in press.
- Bauer TM et al., Brain penetration of lorlatinib and cumulative incidence rates for CNS and non-CNS progression from a phase 1/2 study. WCLC 2018, MA08.05
- Arrieta O et al., Prophylactic cranial irradiation reduces the risk of brain metastases in high-risk lung cancer patients: EGFR and ALK mutations. WCLC 2018, MA08.02
- Arrieta O et al., Effect of prophylactic cranial irradiation on cognitive function and QoL in NSCLC patients at high risk of brain metastases. WCLC 2018, P01.01-03
- Geier M et al., Real-life intracerebral efficacy of nivolumab in non-small cell lung cancer patients with brain metastases. WCLC 2018, MA08.10