Immunotherapy: combination regimens and new data on the significance of mutations
KEYNOTE-189 update after 46.3 months
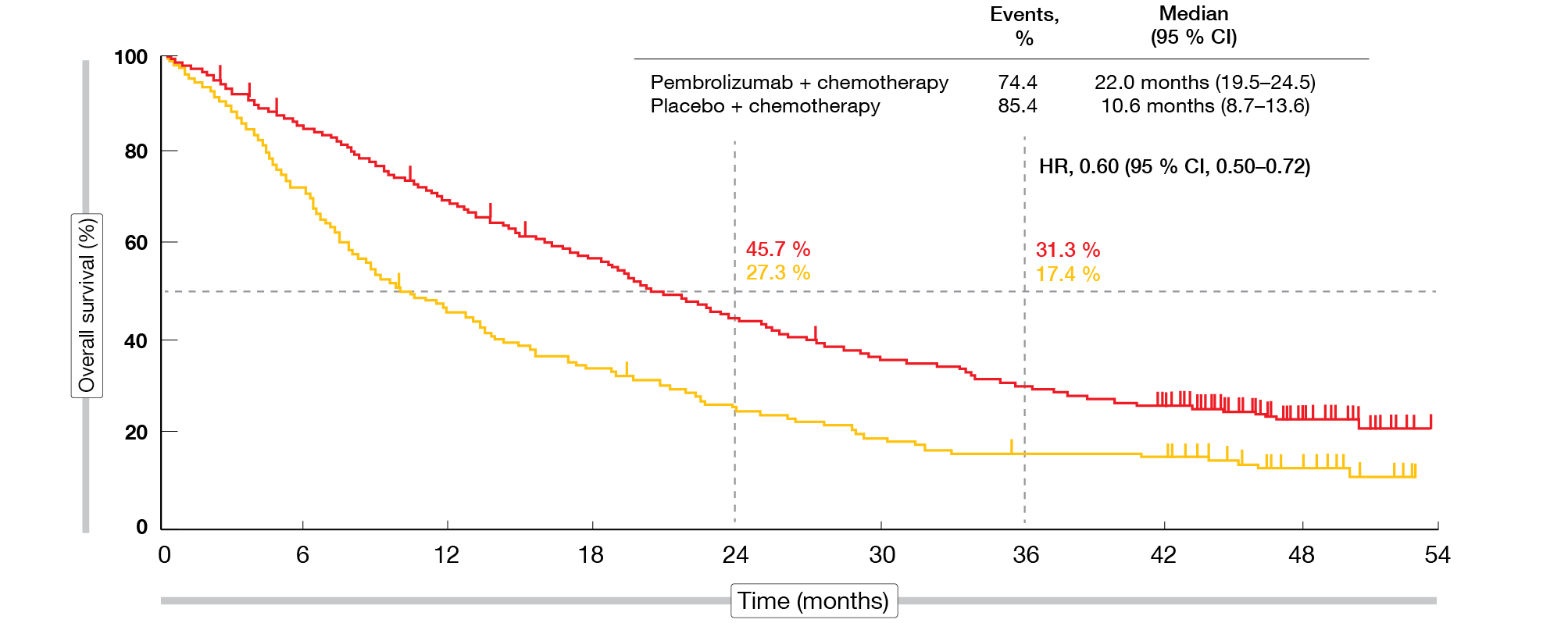
Substantial OS and PFS improvements have led to the implementation of the regimen evaluated in the KEYNOTE-189 trial as standard first-line approach for stage IV non-squamous NSCLC without sensitizing EGFR/ALK alterations [1]. Pembrolizumab plus platinum/pemetrexed for up to 4 cycles followed by pembrolizumab for up to 31 cycles plus pemetrexed (n = 410) was tested against placebo plus platinum/pemetrexed followed by placebo plus pemetrexed (n = 206). Risk reductions of approximately 50 % were achieved with the immunotherapy-based strategy for both OS and PFS (HRs, 0.49 and 0.52, respectively). After a median follow-up of 46.3 months, Gray et al. presented updated efficacy and safety outcomes for the overall study population (intent-to-treat, ITT) as well as for patients who completed 35 cycles, i.e., 2 years of pembrolizumab (n = 56) [2].
Pembrolizumab plus platinum/pemetrexed continued to provide OS and PFS benefits compared to placebo plus chemotherapy, while showing a manageable safety profile. Median OS was 22.0 vs. 10.6 months in the ITT population, with the 3-year OS rate being almost doubled in the experimental arm (31.3 % vs. 17.4 %; HR, 0.60; Figure 1). Median PFS was 9.0 vs. 4.9 months (HR, 0.50). At 36 months, 11.8 % vs. 1.3 % of patients were progression-free. OS and PFS benefits emerged irrespective of PD-L1 baseline expression. PFS2, which was defined as the time from randomization to investigator-assessed disease progression that led to cessation of second-line therapy, start of third-line therapy, or death, was 17.0 vs. 9.0 months (HR, 0.52). The ORR amounted to 48.3 % vs. 19.9 %, and responses lasted for a median of 12.6 vs. 7.1 months.
In the group of patients who completed 35 cycles of pembrolizumab, the 2-year OS rate from completion of this treatment was 79.6 %. Objective responses occurred in 87.5 %, with complete responses resulting in 10.7 %. Forty-five patients (80.4 %) were alive at data cut-off; 28 of them showed no signs of disease progression.
Figure 1: Sustained overall survival benefit for pembrolizumab plus chemotherapy vs. placebo plus chemotherapy: KEYNOTE-189
Pembrolizumab plus ipilimumab: KEYNOTE-598
Negative results were obtained in the KEYNOTE-598 study for pembrolizumab plus ipilimumab as first-line therapy of patients with stage IV NSCLC and PD-L1 TPS ≥ 50 % who had no targetable EGFR/ALK alterations [3]. KEYNOTE-598 tested this approach based on the fact that dual immunotherapy with the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab is a standard of care for advanced melanoma and renal cell carcinoma [4, 5]. Appropriately powered, controlled comparisons of anti–PD-1 monotherapy versus dual PD-1 and CTLA-4 inhibition as first-line treatment for NSCLC had been lacking to date. The KEYNOTE-598 study included 284 patients in each arm; those in the experimental arm received pembrolizumab for up to 35 doses plus ipilimumab for up to 18 doses, while those in the control arm were treated with pembrolizumab plus placebo.
However, adding ipilimumab to pembrolizumab did not improve efficacy compared with pembrolizumab alone. No differences were observed regarding OS (21.4 vs. 21.9 months; HR, 1.08; p = 0.74), PFS (8.2 vs. 8.4 months; HR, 1.06; p = 0.72), ORR (45.4 % in both arms), and duration of response (16.1 vs. 17.3 months). KEYNOTE-598 was stopped for futility based on the recommendation of the data monitoring committee. Moreover, the combination of pembrolizumab and ipilimumab gave rise to greater toxicity than pembrolizumab alone. As the authors summarized, pembrolizumab monotherapy remains a standard-of-care first-line treatment for NSCLC patients with TPS ≥ 50 % who do not harbor EGFR/ALK aberrations.
KRAS & TP53 mutations: predicting IO efficacy
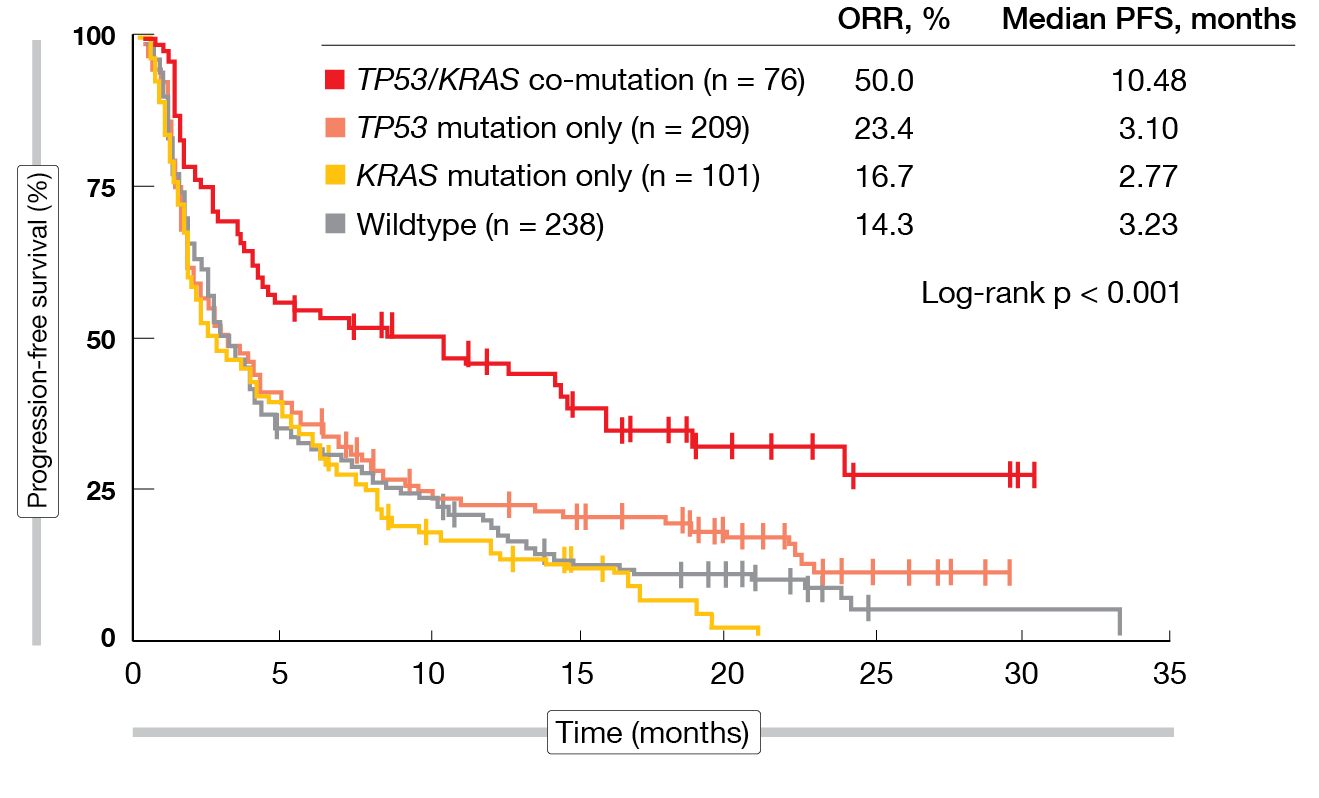
Genetic aberrations in the KRAS and TP53 genes are common in NSCLC. Using a meta-cohort analysis of 8 cohorts including a total of 1,129 patients, Li et al. investigated the interrelation between these two gene mutations with respect to the prediction of immune checkpoint inhibition efficacy in EGFR/ALK wildtype, non-squamous NSCLC [6].
TP53 mutations were shown to be associated with higher ORR and PFS in patients with KRAS mutations, but not in the KRAS wildtype population. Conversely, KRAS mutations were associated with better ORR and PFS in TP53-mutant but not in TP53-wildtype patients. TP53–KRAS co-mutations predicted longer PFS on immunotherapy whereas either TP53 or KRAS mutations alone did not (Figure 2). In chemotherapy-treated patients, the presence of the TP53–KRAS co-mutation had no effect on PFS. The co-mutation was demonstrated to predict benefit from atezolizumab over docetaxel, regardless of tumor mutational burden, PD-L1 expression, significant clinicopathological features, and immunotherapy-related mutational events.
In their conclusion, the authors emphasized the interdependence of KRAS and TP53 mutations in predicting the benefit of immune checkpoint inhibition. Considering this, future researchers probing into predictors of immunotherapy were advised to focus on the mutual influence between distinct biomarkers.
Figure 2: Progression-free survival in immunotherapy-treated patients: TP53/KRAS co-mutation vs. TP53 and KRAS single mutations and wildtype
No nivo/ipi benefit in EGFR-mutant disease
In patients with EGFR-mutant NSCLC, monotherapy PD-1 inhibition has been shown to be associated with low clinical efficacy [7, 8]. Lai et al. conducted an open-label, randomized, phase II study to test combination immune checkpoint inhibition in patients with advanced EGFR-mutant NSCLC who had failed one line of standard EGFR TKI treatment and ≤ 1 line of chemotherapy [9]. Arm A received nivolumab monotherapy (n = 15), while Arm B was treated with nivolumab plus ipilimumab (n = 16). Treated or stable metastases were allowed. On disease progression, patients from Arm A could cross over to Arm B. Biomarker evaluations including exome sequencing and plasma cytokine analysis, among others, were part of the proceedings.
Combined immune checkpoint inhibition did not result in clinical benefit. The study was terminated early after 31 patients due to futility. An ORR of 3.2 % was observed in the overall cohort, with one patient attaining partial response in the combination arm and none responding in the monotherapy arm. Stable disease occurred in 6 patients each (40.0 % and 37.5 %, respectively). PFS was similar across the groups (median, 1.31 and 1.22 months, respectively). Five patients derived clinical benefit in the form of ongoing partial response/stable disease at 6 months or best response of partial response. All of these had EGFR exon 19 deletion, and one had a T790M mutation. No association existed between PD-L1 status and response to immune checkpoint inhibition. None of the three patients who progressed and crossed over from monotherapy to combination therapy achieved salvage. The use of immunotherapy did not result in increased safety concerns. Immune-related AEs occurred with similar rates as observed in the CheckMate 227 trial [10].
According to the biomarker analysis, tumor mutational burden was generally low, even in patients who achieved clinical benefit. Likewise, no clear pattern became evident between the Gene Expression Profiling Test (GEP) score and response to immunotherapy. However, patients who derived clinical benefit were either “immune-hot” at baseline or became “hot” on treatment. Additionally, they tended to have lower numbers of myeloid-derived suppressor cells over time. The authors noted that lack of intracranial control might have been a major factor contributing to the poor outcomes in this study. Intracranial failure should therefore be an important consideration when choosing immunotherapy for EGFR-mutant NSCLC patients. Combinatorial approaches might be a solution, although further research is required.
Nivolumab plus MET inhibition
Concurrent treatment with the MET inhibitor capmatinib and the PD-1 inhibitor nivolumab was assessed in a study based on the rationale that dysregulation of the MET pathway might modulate the immune cell function, leading to suppression of anticancer immune responses [11]. In mouse models, capmatinib has been shown to enhance the efficacy of immunotherapy irrespective of tumor-cell–intrinsic MET dependence [12]. The multicenter, global, open-label, phase II trial reported at WCLC included patients with advanced/metastatic, EGFR-wildtype, PD-(L)1-inhibitor–naïve NSCLC and documented disease progression after standard-of-care treatment [13].
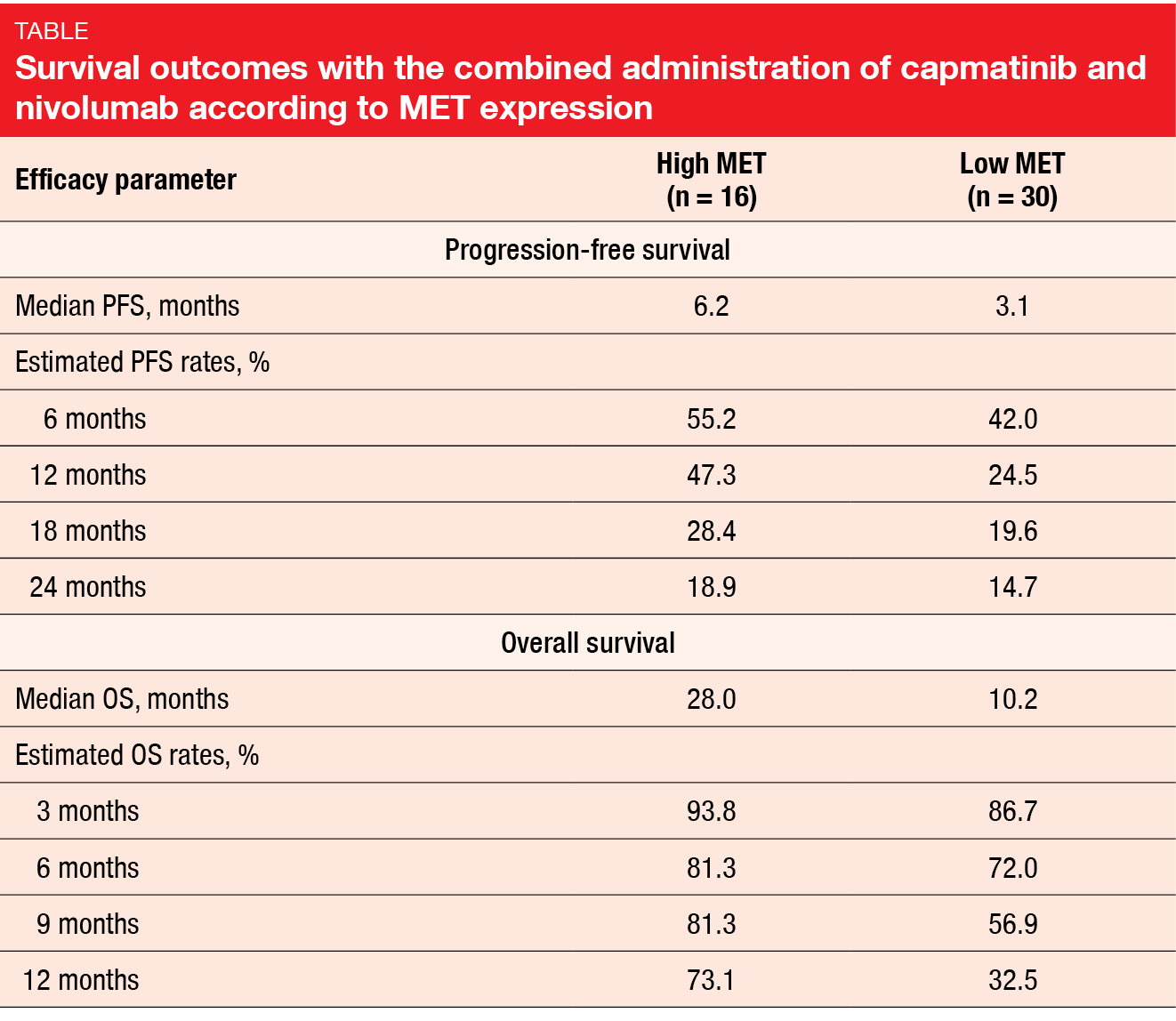
Capmatinib 400 mg BID was administered together with nivolumab 3 mg/kg every 2 weeks. The patients were stratified according to MET expression, with 16 individuals meeting high-MET criteria (i.e., MET IHC 3+ in ≥ 50 % of tumor cells regardless of gene copy number [GCN], or IHC 2+ in ≥ 50 % of tumor cells and GCN ≥ 5, or METex14-positive disease) and 30 patients meeting low-MET criteria (i.e., METex14-negative or unknown status and one of the following: MET IHC 2+ in ≥ 50 % of tumor cells and GCN < 5; IHC 2+ in < 50 % of tumor cells regardless of GCN; IHC 0 or 1+ regardless of GCN). PFS at 6 months was defined as the primary endpoint. Felip et al. presented the results after a median follow-up of 22.9 and 30.4 months for the high-MET and low-MET cohorts, respectively [13].
This first report showed clinical activity of capmatinib plus nivolumab in both high-MET and low-MET patients. ORRs were 25.0 % and 16.7 %, respectively, with disease control rates of 81.3 % and 40.0 %, respectively. Median duration of response was 22.89 and 24.99 months, respectively. Tumor shrinkage occurred in both high-MET and low-MET patients. Median PFS was 6.2 and 3.1 months, respectively, with 6-month PFS rates of 55.2 % and 42.0 %, respectively (Table). Median OS had been reached after 28.0 and 10.2 months, respectively. At 12 months, 73.1 % and 32.5 % of patients were alive. The authors noted that in this study with a limited sample size, the endpoints numerically favored capmatinib plus nivolumab in the high-MET group over the low-MET group, with overlapping 95 % confidence intervals.
The combination showed a manageable safety profile. Among grade 3/4 treatment-related AEs, increases in amylase (15.2 %) and lipase (10.9 %) were most commonly observed, followed by vomiting (8.7 %), nausea, asthenia, and peripheral edema (6.5 % each). Treatment-related AEs led to study drug discontinuation in 39.1 % and 19.6 %, respectively, across the two cohorts. Dose adjustments or interruptions became necessary in 80.4 % and 60.9 %, respectively, and additional therapy due to AEs was required in 93.5 % and 58.7 %, respectively.
Anti-angiogenic combination partner
The chemotherapy-free combination of the anti–PD-1 antibody sintilimab and the multi-target anti-angiogenic TKI anlotinib was evaluated in a phase I study conducted in treatment-naïve patients with stage IIIB/IV NSCLC without driver aberrations. The primary analysis has shown encouraging ORR regardless of PD-L1 expression [14]. At WCLC 2020, Han et al. reported the final analysis for the primary endpoints that demonstrated durable efficacy and good tolerability [15]. Overall, 72.7 % of patients (n = 22) responded, with 100 % achieving disease control. Median duration of response had not been reached yet. Median PFS was 15 months, while OS data were still immature. At 12 months, 95.5 % of patients were alive, and 71.4 % were progression-free.
Responses and freedom from progression occurred irrespective of tumor mutational burden, PD-L1 expression, or histology. With longer follow-up, sintilimab plus anlotinib did not give rise to any unexpected toxicities. The authors pointed out that this novel combination offers potential efficacy for a broader range of NSCLC patients regardless of histologic subtype or PD-L1 status. A randomized phase II trial is currently ongoing to further investigate sintilimab plus anlotinib (NCT04124731).
Long-term AEs in long-term survivors
Hsu et al. presented a retrospective analysis of immunotherapy survivors, i.e., patients with stage III/IV NSCLC alive at > 1 year after the initiation of PD-(L)1 treatment [16]. Overall, 317 patients treated between November 2009 and February 2020 were identified. In this group, 114 (36 %) had survived for > 1 year. Approximately half of them were aged ≥ 65 years, and > 80 % were current or former smokers. Adenocarcinoma prevailed as the most common histology in 66 %. Sixty-one percent had unknown PD-L1 status; in 21 % PD-L1 expression exceeded 50 %. The median number of doses received was 13 (range, 1-121). Two thirds and one third of patients had received monotherapy and combination therapy, respectively.
Immune-related AEs (irAEs) occurred in 59 long-term survivors (52 %), with pulmonary events (pneumonitis) and dermatologic events (dermatitis, pruritus, psoriasis) emerging most commonly, followed by endocrine (hypothyroidism, thyroiditis, hypophysitis, type 1 diabetes, fatigue), rheumatologic (inflammatory arthritis, Sicca syndrome, xerostomia, dry eye, costochondritis) and gastrointestinal (colitis, diarrhea, hepatitis, pancreatitis) AEs. Among the 59 affected patients, 39 had a single irAE, whereas 20 developed more than one. The most common multi-system irAEs included combinations of pneumonitis with dermatitis (n = 4), inflammatory arthritis (n = 3), and Sicca syndrome (n = 2). Median time to single and multi-system irAEs was 22 and 9 weeks, respectively. The authors noted that 31 (27 %) of long-term survivors required ongoing irAE management at one year. Overall, NSCLC survivors treated with immune checkpoint inhibition represent a group with unique long-term needs.
REFERENCES
- Gandhi L et al., Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 387(22): 2078-2092
- Gray JE et al., Pembrolizumab + pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. WCLC 2020, PF13.02
- Boyer M et al., Pembrolizumab plus ipilimumab vs pembrolizumab plus placebo as 1L therapy for metastatic NSCLC of PD-L1 TPS ≥ 50 %: KEYNOTE-598. WCLC 2020, PS01.09
- Larkin J et al., Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1): 23-34
- Motzer RJ et al., Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277-1290
- Li X et al., Interdependence of KRAS and TP53 mutations in predicting ICI efficacy in EGFR/ALKWT non-squamous NSCLC: results from 1,129 patient-level data. WCLC 2020, OA07.06
- Lisberg A et al., A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol 2018; 13(8): 1138-1145
- Gainor J et al., EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22(18): 4585-4593
- Lai GGY et al., Randomised phase 2 study of nivolumab versus nivolumab and ipilimumab combination in EGFR mutant NSCLC. WCLC 2020, OA01.06
- Hellmann MD et al., Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019; 381(21): 2020-2031
- Papaccio F et al., HGF/MET and the immune system: relevance for cancer immunotherapy. Int J Mol Sci 2018; 19(11): 3595
- Glodde N et al., Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 2017; 47(4): 789-802.e9
- Felip E et al., Efficacy and safety of capmatinib plus nivolumab in pretreated patients with EGFR wild-type non-small cell lung cancer. WCLC 2020, FP76.03
- Han BH et al., Efficacy and safety of sintilimab with anlotinib as first-line therapy for advanced non-small cell lung cancer. WCLC 2019, JCSE01.11
- Han B et al., Sintilimab in combination with anlotinib as first-line therapy for advanced NSCLC: final analysis of primary endpoints. WCLC 2020, OA07.09
- Hsu M et al., Survivors from anti-PD-(L)1 immunotherapy in NSCLC: clinical features, survival outcomes and long-term toxicities. WCLC 2020, MA07.05
© 2020 Springer-Verlag GmbH, Impressum
More posts
Nivolumab as a new option in patients with relapsed malignant mesothelioma
Nivolumab as a new option in patients with relapsed malignant mesothelioma Unti
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Immunotherapy: combination regimens and new data on the significance of mutations
Immunotherapy: combination regimens and new data on the significance of mutations
What is new in SCLC?
What is new in SCLC? Lurbinectedin plus irinotecan A novel approach for targeti
Interview: Antibody-drug conjugates: the age of almost unlimited possibilities has just begun
Interview: Antibody-drug conjugates: the age of almost unlimited possibilities has
Specific treatment approaches in the EGFR-mutated setting
Specific treatment approaches in the EGFR-mutated setting Exon 20 insertions: p