New approaches in relapsed and refractory DLBCL
Polatuzumab vedotin plus R-ICE
Approximately one third of patients with diffuse large B-cell lymphoma (DLBCL) develop relapsed or refractory disease, which remains a major cause of mortality [1]. In patients relapsing more than 1 year after first-line treatment, the standard of care is salvage treatment followed by autologous stem cell transplantation (ASCT), although responses to platinum-based salvage therapy are generally suboptimal. The CD79b-targeting antibody-drug conjugate polatuzumab vedotin was tested in the setting of relapsed/refractory DLBCL together with R-ICE (i. e., rituximab, ifosfamide, carboplatin, etoposide) as first salvage therapy based on the hypothesis that PolaR-ICE would be a safe and effective bridge to ASCT. At ASH 2022, Herrera et al. presented findings for 41 transplant-eligible patients with primary refractory (n = 20) or relapsed (n = 21) DLBCL after 1 prior anthracycline-based therapy [2].
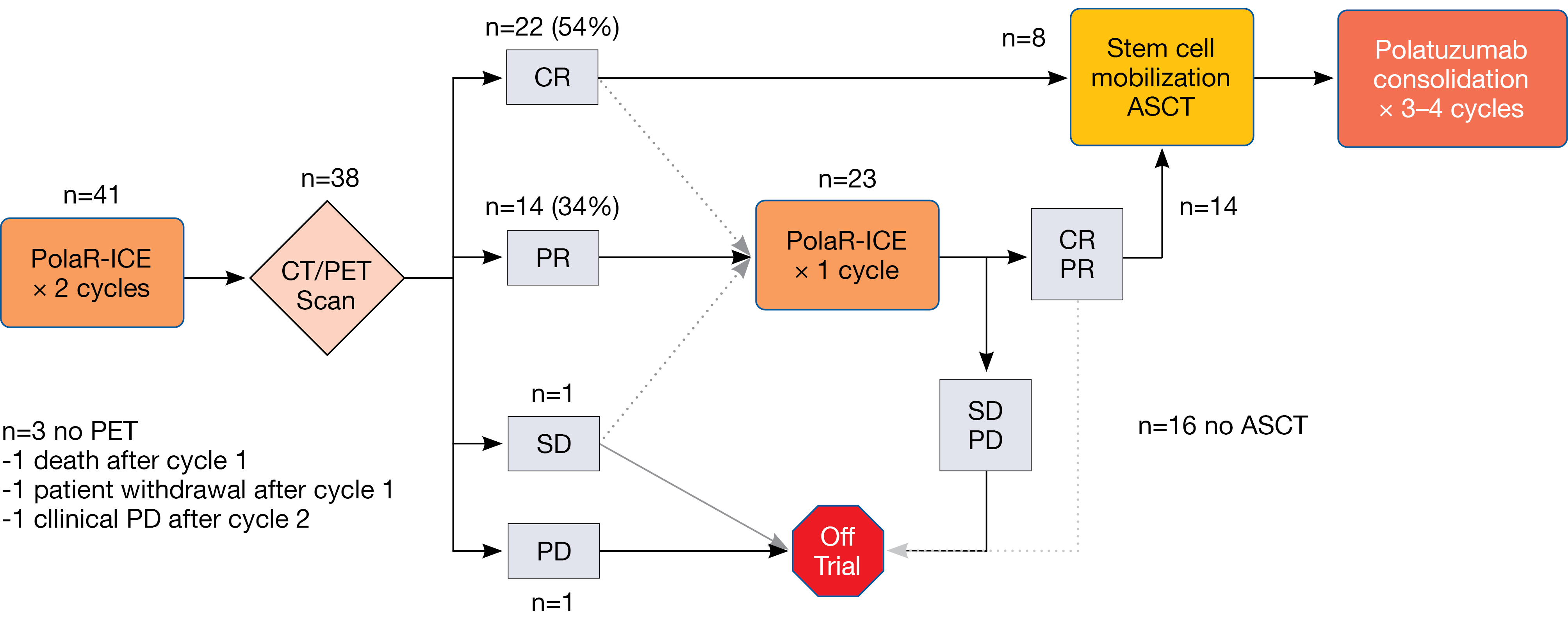
According to the study schema, CT/PET staging was performed after 2 cycles of PolaR-ICE. Patients who obtained complete response (CR) or partial response (PR) could directly proceed to ASCT or undergo ASCT after they had received a third cycle of PolaR-ICE; this was at the discretion of the treating investigator. ASCT was followed by polatuzumab vedotin consolidation for 3–4 cycles. The maximum overall number of polatuzumab vedotin cycles was 6. Primary endpoints included safety in the lead-in phase that established the recommended phase II dose of polatuzumab vedotin at 1.8 mg/kg, as well as CR after 2 cycles of PolaR-ICE.
Bridging to ASCT in 61 %
The safety lead-in identified no dose-limiting toxicities. After 2 cycles, 22 of 38 patients (54 %) amenable to staging had developed CR (Figure). Together with a PR rate of 34 %, this made for an overall response rate (ORR) of 88 %. Twenty-three patients (56 %) received 3 cycles of PolaR-ICE. At the end of salvage, ORR and CR rates were 80 % and 56 %, respectively. Overall, 22 patients (61 %) underwent ASCT, and 16 (39 %) proceeded to polatuzumab vedotin consolidation. In 10.7 %, stem cell collection failure occurred. The impact of polatuzumab vedotin on stem cell collection remained unclear, as the authors noted. Patients with relapsed DLBCL had higher CR rates than those with primary refractory disease both after 2 cycles (76 % vs. 30 %) and at the end of salvage (76 % vs. 35 %). Preliminary analyses revealed a similar picture regarding PFS, although longer follow-up is required to assess this endpoint adequately. After a median follow-up of 6.6 months, median PFS had not been reached in patients who went on to receive ASCT.
PolaR-ICE was shown to be a tolerable first salvage treatment option. Anemia, decreased platelet counts, and nausea were the most common attributable adverse events (AEs). The authors pointed out that a bridging rate of 61 % compares favorably with historical results. It appears that in relapsed DLBCL, PolaR-ICE is a potent salvage regimen that should be considered for further study.
Figure: Responses to PolaR-ICE after 2 cycles and at the end of salvage treatment
Zanubrutinib/lenalidomide: promising preliminary results
The ongoing open-label, multicenter, phase I dose-escalation and dose-expansion study BGB-3111-110 is investigating the next-generation BTK inhibitor zanubrutinib in addition to lenalidomide in Chinese patients with relapsed/refractory DLBCL after ≥ 1 line of systemic therapy. The patients were ineligible for high-dose therapy/stem cell transplantation if they had not received this previously. Zanubrutinib 160 mg BID was administered together with one of three doses of lenalidomide (i. e., 15 mg QD, 20 mg QD or 25 mg QD on days 1-21) in the dose escalation part. Preliminary results from this part of the study (n = 27) were reported at ASH 2022 by Zhang et al. [3]. The population included a total of 27 patients, with 6, 10, and 11 individuals being treated at the lenalidomide dose levels 1, 2, and 3, respectively. Seventy-four percent had stage III/IV disease, and 52 % had refractory DLBCL.
The ORR identified in the overall cohort was 51.9 %, and CR was achieved in 22.2 %. Patients treated at dose level 3 showed the best ORR of 90.9 % and the highest CR rate of 36.4 %. Median duration of response had not been reached at the time of the analysis. Median PFS was 5.52 months in the overall group; for dose levels 1, 2, and 3, this was 2.79 months, 2.89 months, and not reached, respectively.
At the same time, zanubrutinib plus lenalidomide showed an acceptable safety profile. No patient experienced dose-limiting toxicity at any dose level, and the maximum tolerated dose was not reached. The incidence of any-grade treatment-emergent AEs (TEAEs) did not increase significantly with increasing dose. Grade ≥ 3 TEAEs occurred in 63 %, were mostly hematologic events, and were generally manageable across all dose levels. Based on these efficacy and safety data, the recommended phase II dose of lenalidomide in this combination was determined to be dose level 3, i. e., lenalidomide 25 mg QD on days 1–21 of each 28-day cycle in addition to zanubrutinib 160 mg BID continuously. Patient recruitment for the dose expansion part of the study that uses dose level 3 and will focus on the ORR as the primary endpoint is ongoing.
REFERENCES
- Poletto S et al., Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev 2022; 110: 102443
- Herrera AF et al., Polatuzumab vedotin combined with R-ICE (PolaR-ICE) as second-line therapy in relapsed/refractory diffuse large B-cell lymphoma. ASH 2022, abstract 442
- Zhang H et al., Preliminary safety and efficacy of zanubrutinib in combination with lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma. ASH 2022, abstract 1627
© 2023 Springer-Verlag GmbH, Impressum
More posts
Clinical findings with sundry targets in various B-cell malignancies
Clinical findings with sundry targets in various B-cell malignancies AUGMENT: 5-year r
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches Advanced-stage follicular
New approaches in relapsed and refractory DLBCL
New approaches in relapsed and refractory DLBCL Polatuzumab vedotin plus R-ICE Approxi
Chronic lymphocytic leukemia: moving towards new horizons
Chronic lymphocytic leukemia: moving towards new horizons Final analysis of ALPINE: za
Further steps to improve efficacy and safety in acute myeloid leukemia
Further steps to improve efficacy and safety in acute myeloid leukemia Long-term follo
Active monotherapies and combinations in mantle cell lymphoma
Active monotherapies and combinations in mantle cell lymphoma First-line triple combin