Chronic lymphocytic leukemia: moving towards new horizons
Final analysis of ALPINE: zanubrutinib vs. ibrutinib
The first-in-class, covalent BTK inhibitor ibrutinib has transformed the treatment of patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). However, toxicities frequently lead to treatment discontinuation [1-4]. Moreover, exposure coverage between dosing intervals falls below the IC50 threshold, and BTK occupancy at trough levels is variable [5]. The second-generation BTK inhibitor zanubrutinib has been designed to have greater BTK specificity than ibrutinib, with exposure coverage above its IC50 [6]. More sustained and complete BTK inhibition can be expected based on higher drug-concentration/IC50 ratios. In patients with treatment-naïve CLL/SLL without del(17p), zanubrutinib has induced superior progression-free survival (PFS) compared with chemoimmunotherapy [7].
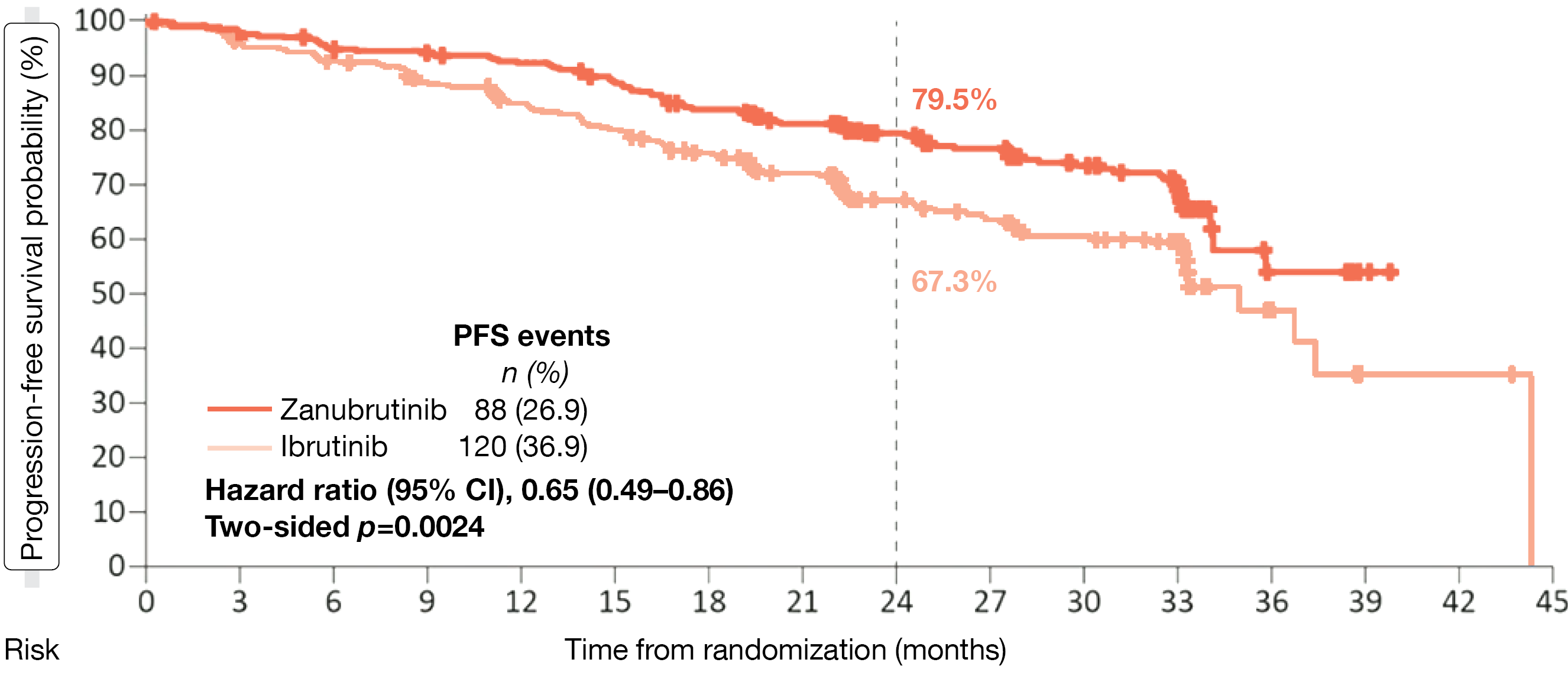
The randomized, phase III ALPINE study evaluated zanubrutinib in the setting of relapsed/refractory (r/r) CLL/SLL after ≥ 1 prior systemic therapy. Patients were randomized to either zanubrutinib 160 mg BID (n = 327) or ibrutinib 420 mg QD (n = 325). Overall response rate (ORR) non-inferiority and superiority as assessed by the investigators were defined as the primary endpoint. Brown et a. presented the final PFS analysis of the ALPINE trial at the ASH 2022 Congress [8]. PFS by independent review was significantly prolonged with zanubrutinib versus ibrutinib after a median follow-up of 29.6 months, resulting in 24-month PFS rates of 79.5 % vs. 67.3 % and a 35 % reduction in the risk of progression or death (HR, 0.65; p = 0.0024; Figure 1). The findings favored the second-generation agent across subgroups including patients with del(17p)/TP53 mutation; here, the 24-month rates were 77.6 % vs. 55.7 % (HR, 0.52; p = 0.134). The ORR according to independent review was 86.2 % vs. 75.7 % (p = 0.0007). Fewer deaths had occurred in the experimental arm, which gave rise to a 24 % mortality reduction (HR, 0.76), although median overall survival (OS) had not been reached yet in either arm.
Compared with ibrutinib, zanubrutinib displayed a favorable safety profile, with lower rates of grade ≥ 3 adverse events (AEs; 67.3 % vs. 70.4 %) and serious AEs (42.0 % vs. 50.0 %). AEs led to dose reduction in 12.3 % vs. 17.0 % and treatment discontinuation in 15.4 % vs. 22.2 %. While hypertension and bleeding events occurred with comparable incidence across the arms, patients treated with zanubrutinib were less likely to experience cardiac AEs (21.3 % vs. 29.6 %) and serious cardiac AEs (1.9 % vs. 7.7 %). Cardiac events prompting treatment discontinuation were noted in 0.3 % vs. 4.3 % and fatal cardiac events in 0 % vs. 1.9 %. Atrial fibrillation/flutter was significantly less commonly observed (5.2 % vs. 13.3 %; p = 0.0004).
In their conclusion, the authors emphasized that ALPINE is the first study to demonstrate PFS superiority in a head-to-head comparison of BTK inhibitors in patients with r/r CLL/SLL. Superiority has now been proven with regard to both PFS and ORR.
Figure 1: ALPINE study: superiority of zanubrutinib vs. ibrutinib regarding progression-free survival
CAPTIVATE: fixed-duration first-line treatment
The MRD cohort of the international, multicenter, phase II CAPTIVATE study assessed fixed-duration versus continued administration of ibrutinib following first-line treatment with ibrutinib plus venetoclax. Patients who had confirmed undetectable minimal residual disease (uMRD) after completion of the combination therapy were randomly assigned in a double-blind manner to placebo (n = 43) or continued ibrutinib (n = 43). At ASH 2022, Allan et al. presented the findings after a median follow-up of 56 months [9].
Forty-one months after completion of treatment with ibrutinib plus venetoclax, 3-year disease-free survival (DFS) rates were similar across the arms (85 % vs. 93 %; HR, 0.435; p = 0.1621). DFS was defined as death from any cause, disease progression, or MRD conversion (MRD >10-4). Likewise, complete response (CR) rates were stable or had increased further over time. Duration of CR did not differ significantly between the treatment arms. MRD negativity was sustained 3 years post-randomization, with the rates being similar across all patients. As the authors noted, the durability of MRD negativity and the 3-year DFS rate of 85 % in the placebo arm further support the potential for durable treatment-free remissions in the majority of patients. At 48 months, PFS was 88 % and 95 % with placebo and continued ibrutinib, respectively. Patients with unmutated IGHV showed PFS rates similar to those in the total population. OS rates remained ≥ 98 % across the ibrutinib and placebo arms. In patients with del(17p), TP53 mutation, or complex karyotype, median DFS, PFS and OS were consistent with the total population, although the sample size was small in the placebo arm.
Post-randomization AEs were generally infrequent. No new atrial fibrillation events occurred in the placebo arm, while one such event was observed in the ibrutinib arm. Grade ≥ 3 hemorrhages were seen in neither arm. The authors concluded that the first-line combination of ibrutinib and venetoclax is an all-oral, once-daily, chemotherapy-free regimen with a favorable risk-benefit profile that continues to provide deep, durable responses.
Acalabrutinib, venetoclax & obinutuzumab
Therapeutic triplet regimens are evaluated in CLL based on a strong rationale indicating synergy between BTK inhibition and Bcl-2 inhibition on one hand and CD20-targeted treatment on the other [10-12]. However, TP53-aberrant CLL remains an area of unmet need.
Acalabrutinib plus venetoclax and obinutuzumab (AVO) is being tested in a treatment-naïve all-comer population in the phase II setting. The analysis of this group (n = 37) showed a high uMRD rate of 86 % and a favorable toxicity profile [13]. Notably, uMRD outcomes in the bone marrow were similar regardless of TP53 status.
Therefore, the researchers hypothesized that AVO would be an effective and well-tolerated novel-agent–only, time-limited frontline regimen for high-risk CLL, and opened an expansion cohort including 31 patients with TP53-aberrant disease only (i.e., del[17p] or TP53 mutation). Prior to the initiation of obinutuzumab in cycle 2, patients received one cycle of acalabrutinib alone. Venetoclax was added at cycle 4. Obinutuzumab was administered for 6 cycles, venetoclax for 12 cycles, and acalabrutinib for 15 cycles. At cycle 16, the primary endpoint was assessed, which was CR with uMRD in the bone marrow. Patients who achieved uMRD CR could discontinue therapy, while those who did not received another 9 cycles of acalabrutinib plus venetoclax. Restaging was performed at cycle 25, and patients again had the option to discontinue therapy if they had achieved uMRD. At ASH 2022, Ryan et al. presented longer-term follow-up data from the initial all-comer cohort and results from the expansion cohort [14].
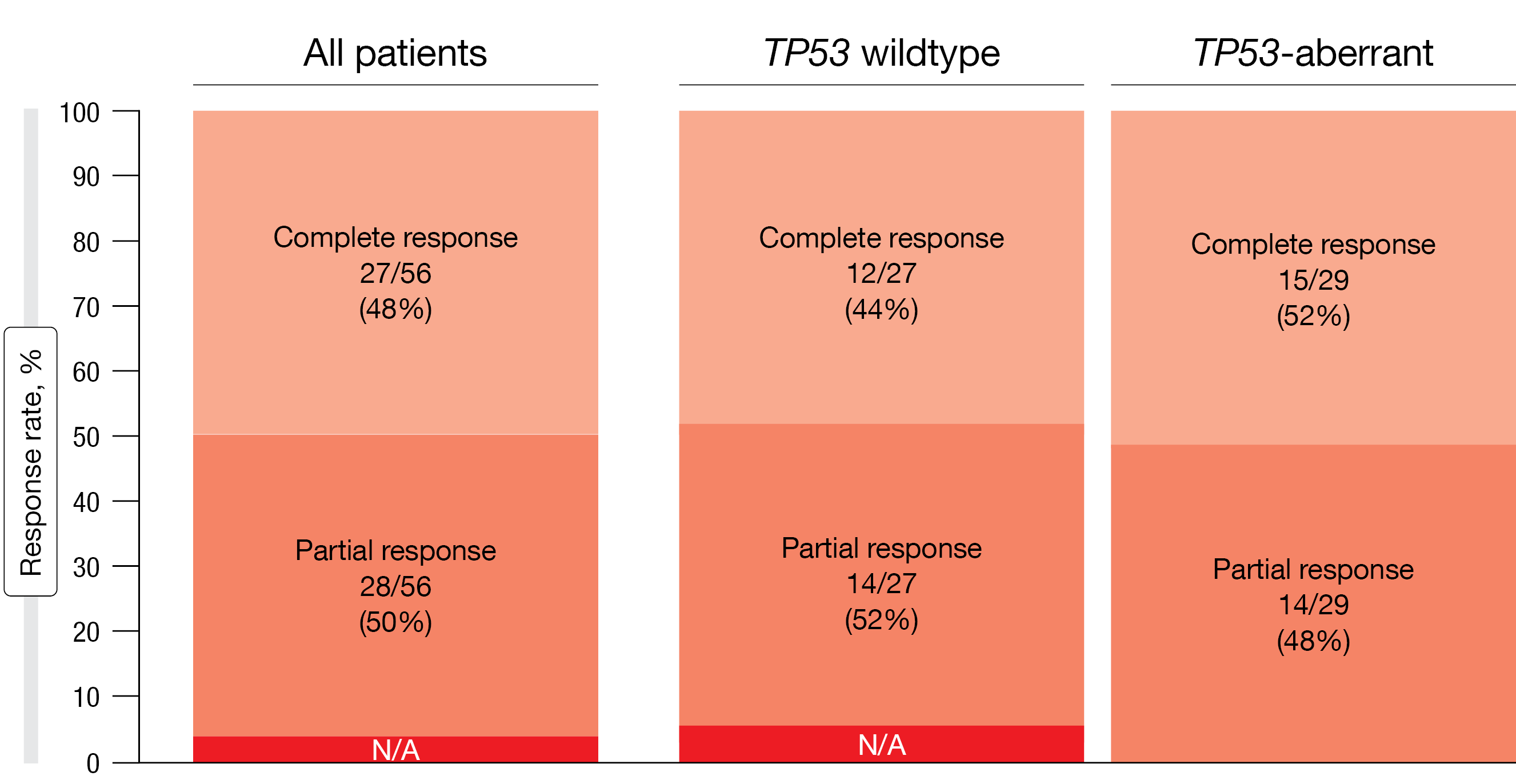
In the entire group, 43 % of patients achieved the primary endpoint, which was CR with uMRD in the bone marrow at cycle 16. For the TP53-aberrant group, this was 45 %. AVO induced pronounced clinical responses, with a CR rate of 48 % in the total population (Figure 2). Subgroup analyses showed similar CR rates irrespective of the TP53 mutation status. uMRD was identified in 86 % in both peripheral blood and bone marrow in all patients. Again, the results were consistent across the TP53-wildtype and TP53-aberrant groups. In patients who had reached cycle 25 on study, similar findings to those seen at cycle 16 emerged; the CR rate was 44 %, and uMRD rates were 89 % and 84 % in the peripheral blood and bone marrow, respectively. MRD assessment via clonoSEQ® next-generation sequencing demonstrated durable MRD negativity in the blood, with uMRD < 10-5 in 59 % and 61 % at cycles 16 and 25, respectively.
Figure 2: Acalabrutinib plus venetoclax and obinutuzumab: high clinical response rates at cycle 16 by iwCLL criteria
Lasting treatment effects
Among 43 patients who electively discontinued therapy after achieving uMRD in the bone marrow, 22 remained in CR or partial remission (PR) at cycle 24 after a median time off therapy of 18.8 months. Four individuals who electively discontinued therapy after 15 cycles had disease recurrence, with three showing MRD recurrence only and one patient with overt CLL disease progression. Overall, at a median follow-up of 35 months, 92.6 % of all patients were alive and progression-free.
The safety profile contained the expected toxicities of the three agents, including headache, fatigue, and bruising. Most of these were grade 1 or 2. Thirty-seven percent of patients had grade 3/4 neutropenia; however, no patient developed febrile neutropenia or opportunistic infections. Relatively low rates of grade ≥ 3 infections were observed, although one patient died of COVID-19 pneumonia. Two cases of atrial fibrillation occurred. Fourteen patients (21 %) required any dose reduction.
In their summary, the scientists stated that AVO is a highly active, well tolerated triplet regimen in a frontline CLL population enriched for high-risk disease. These findings provide a foundation for the MRD-guided, time-limited triplet therapy, particularly in high-risk CLL. AVO is currently being tested against acalabrutinib/venetoclax and chemoimmunotherapy in the phase III ACE-CL-311/AMPLIFY trial in non–high-risk CLL (NCT03836261).
GIVe regimen in high-risk patients
Another triplet is obinutuzumab, ibrutinib, and venetoclax (GIVe) that was assessed in previously untreated patients with del(17p)/TP53 mutation in the phase II CLL2-GIVe trial. During the induction phase, obinutuzumab was administered for 6 cycles together with ibrutinib and venetoclax; the consolidation phase included ibrutinib plus venetoclax through cycle 12, after which ibrutinib continued for 3 more cycles. Patients who achieved uMRD at cycles 9 and 12 as well as CR or CR with incomplete count recovery (CRi) at cycle 15 could discontinue ibrutinib therapy at that time. Those who did not went on to receive ibrutinib for a maximum of 36 cycles. The CR rate at cycle 15 was defined as the primary endpoint.
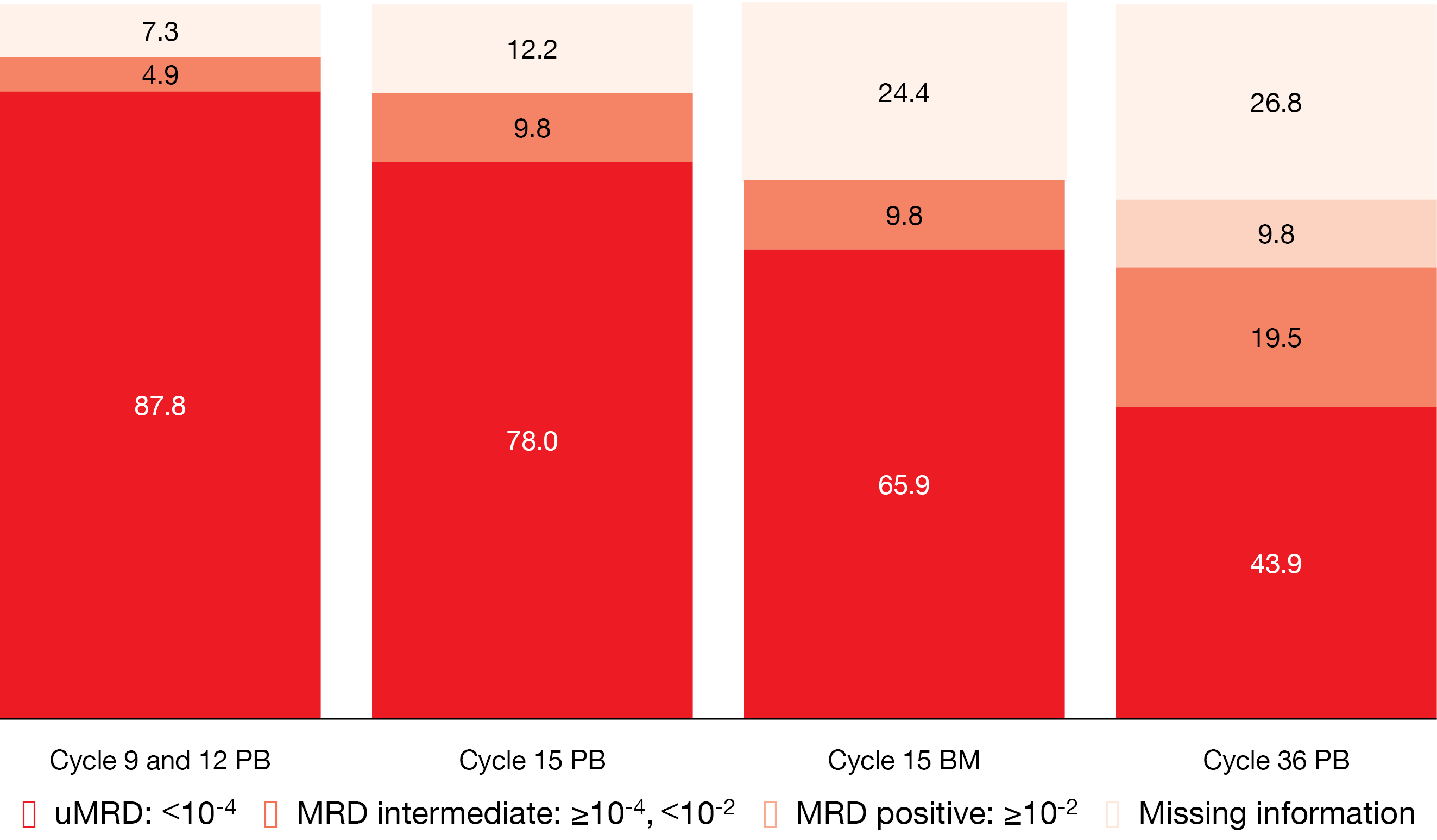
According to the final analysis for 41 patients reported at ASH 2022 by Huber et al., time-limited therapy with the GIVe regimen represents a promising approach in high-risk patients [15]. At the time of the final restaging at cycle 15, all patients responded, with 58.5 % and 41.5 % achieving CR/CRi and PR, respectively. uMRD in the peripheral blood was present in 87.8 % at cycles 9 and 12, and in 78.0 % at cycle 15 (Figure 3). At cycle 36, this proportion had decreased to 43.9 %, although 26.8 % of samples were not assessable at that time due to omitted study visits during the COVID-19 pandemic. The 36-month PFS and OS rates amounted to 79.9 % and 92.6 %, respectively.
The most common hematologic AEs observed in the study included neutropenia (grade ≥ 3, 48.8 %) and thrombocytopenia (grade ≥ 3, 17.1 %). Among non-hematologic AEs, infections occurred in > 80 % of patients and mainly comprised respiratory tract (33 %), pathogen-unspecified (24 %) as well as gastrointestinal and viral infections (9 % each). Approximately 20 % of infections were classified as grade ≥ 3. Atrial fibrillation constituted the most common cardiac AE (14.6 %; grade ≥ 3, 2.4 %). Any-grade AEs and grade ≥ 3 AEs emerged predominantly during induction therapy and decreased during consolidation and maintenance. The authors emphasized the importance of monitoring for infections and supportive measures, which might limit the utility of this regimen in younger and fitter patients. The GIVe regimen is being further assessed in the CLL13/GAIA trial that is comparing GIVe to chemoimmunotherapy, venetoclax/rituximab and venetoclax/obinutuzumab in patients without TP53 deletion or mutation.
Figure 3: Minimal residual disease results for obinutuzumab plus ibrutinib and venetoclax in patients with del(17p)/TP53 mutation. PB, peripheral blood; BM, bone marrow
Phase I data for Bcl-2 inhibitor lisaftoclax
The novel Bcl-2 inhibitor lisaftoclax has been shown to be active in r/r CLL/SLL in a global phase I study, which included patients with TP53 aberrations and/or progression after BTK inhibition [16]. Davids et al. reported the first analysis of a global phase I/II study investigating lisaftoclax as single agent (part 1; n = 46) or in combination (part 2) with rituximab (n = 9) or acalabrutinib (n = 9) in patients with r/r or treatment-naïve CLL/SLL [17]. The dose expansion part contained 100 additional patients who were treated with rituximab plus lisaftoclax (n = 30) or acalabrutinib plus lisaftoclax (n = 70). Overall, 46 patients received lisaftoclax monotherapy, while 39 and 79 were treated with the rituximab and acalabrutinib combinations, respectively. Each study part examined 3 lisaftoclax dose levels of 400 mg, 600 mg, and 800 mg.
Daily lisaftoclax ramp-up within a week was demonstrated to be feasible both in the monotherapy and combination settings. No dose-limiting toxicities have occurred to date. According to pharmacokinetic analyses, the drug exposure levels were dose-dependent and comparable between single agent and combination therapy. There were no significant drug-drug interactions between lisaftoclax and rituximab or acalabrutinib. The most common AEs included neutropenia, diarrhea, and COVID-19 infection. Grade 3/4 neutropenia occurred in 21.0 % to 30.3 % across the groups. Cytopenias generally abated from cycle 3 onward. Clinical tumor lysis syndrome (TLS) was observed in 2.5 % but fully resolved.
Lisaftoclax proved to be active across the range of doses assessed. Almost all patients experienced decreases in lymph node diameters and absolute lymphocyte counts. In the r/r setting, ORRs were 67.4 % for the monotherapy as well as 79.4 % and 98 % for the rituximab and acalabrutinib combinations, respectively. In the treatment-naïve group receiving lisaftoclax plus acalabrutinib, all patients responded. A registrational, phase III study evaluating lisaftoclax plus acalabrutinib in patients with r/r CLL is being planned.
BGB-11417 with and without zanubrutinib
The combination of ibrutinib and the Bcl-2 inhibitor venetoclax has proven effective in patients with CLL/SLL, although toxicities limit its use [18], thus leaving room for improvement in terms of more tolerable combination regimens. In vitro, the novel Bcl-2 inhibitor BGB-11417 has shown more potent and selective Bcl-2 inhibition and improved activity against Bcl-2 mutations than venetoclax [19]. Preliminary data from a first-in-human, multicenter, phase I study assessing BGB-11417 as monotherapy or together with zanubrutinib in patients with CLL/SLL were reported at ASH 2022 by Cheah et al. [20]. Parts 1 and 3 of the study investigated dose escalation including five dose levels (40 mg to 640 mg) for BGB-11417 alone and together with zanubrutinib, while parts 2 and 4 were expansions. In the combination cohort, both a weekly ramp-up schedule and an experimental daily ramp-up schedule were used. Among 79 CLL patients, 8 and 71 received BGB-11417 monotherapy and BGB-11417 plus zanubrutinib, respectively.
The treatment was well tolerated. At the time of the analysis, dose escalation continued to 640 mg with only one dose-limiting toxicity; the maximum tolerated dose had not been achieved. Preliminary pharmacokinetics revealed dose dependence throughout the dose range, fast absorption, and a short half-life of approximately 5 hours. The pharmacokinetics were similar with and without zanubrutinib. In the group treated with single-agent BGB-11417, neutropenia, thrombocytopenia, headache and COVID-19 constituted the most common AEs, while for the combination, this was contusion, COVID-19, diarrhea, and neutropenia. Grade ≥ 3 neutropenia and grade ≥ 2 diarrhea were uncommon and readily manageable. No clinical TLS and a single instance of laboratory TLS occurred. The daily ramp-up group did not develop TLS.
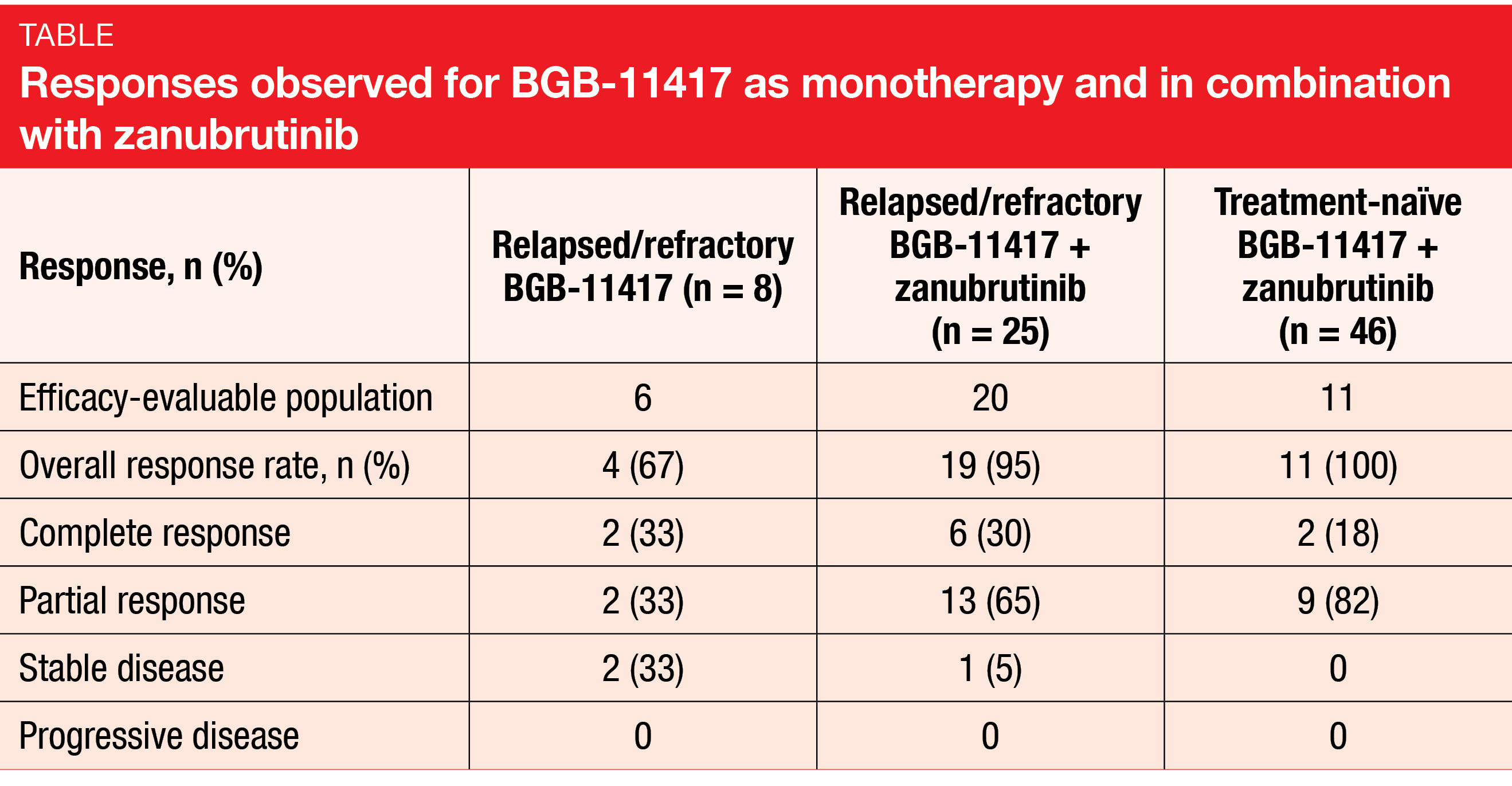
BGB-11417 alone and combined with zanubrutinib demonstrated promising efficacy in patients with r/r and treatment-naïve CLL/SLL. After the weekly ramp-up to 40 mg, absolute lymphocyte counts decreased by approximately 90 % within 6 weeks. The authors stated that this is similar to reductions observed with venetoclax 200 mg [21], theoretically suggesting a five-fold increase in potency. ORRs in efficacy-evaluable patients with r/r disease were 67 % and 95 % for the monotherapy and combination arms, respectively, and 100 % in the treatment-naïve group receiving BGB-11417 plus zanubrutinib (Table). According to the preliminary MRD data, blood MRD negativity was observed at ≥ 80 mg after 6 months in both monotherapy and combination cohorts and increased with longer follow-up and higher dose. Longer observation periods are required to gain further insights here.
MRD-guided duvelisib plus venetoclax
Duvelisib, an oral inhibitor of PI3K-δ/γ, and venetoclax have shown synergistic effects in Richter’s syndrome PDX models [22]. The recommended phase II dose of venetoclax 400 mg QD plus duvelisib 25 mg BID has already been established in a phase I/II study, with promising initial safety and efficacy results [23]. After a 7-day lead-in of duvelisib 25 mg, venetoclax treatment was started. The combination was administered for 12 cycles. On day 1 of cycle 13, restaging was performed; patients with confirmed uMRD were able to discontinue treatment, with a view to resumption of venetoclax therapy in case of MRD recurrence, while those who remained MRD-positive continued on venetoclax. In the phase II, venetoclax was started at doses of 10 mg (outpatient) or 20 mg (inpatient) with weekly ramp-up to 400 mg QD, whereas patients with Richter’s syndrome could undergo accelerated daily ramp-up to 400 mg over 5 days.
Ryan et al. performed an updated analysis of the data for the CLL/SLL cohort (n = 35) and the Richter’s syndrome cohort (n = 8) based on the assumption that duvelisib plus venetoclax will achieve deep remissions with high rates of uMRD, thus allowing for an all-oral, time-limited therapy [24]. Indeed, 12 CLL patients (35 %) achieved CR/CRi with uMRD in the bone marrow after 12 cycles, and 11 electively discontinued therapy. Half of CLL patients with TP53-aberrant disease responded to the treatment. This also applied to the group that had previously received BTK inhibition. At 2 years, 78 % of all CLL patients were progression-free.
Two of the patients who electively discontinued treatment were found to have MRD-only recurrence in the peripheral blood at cycle 25. Both restarted venetoclax monotherapy, and one achieved uMRD. In the group with Richter’s syndrome, two patients (25 %) achieved CR, and one successfully underwent consolidative allogeneic stem cell transplantation. At the time of the analysis, five patients had died due to progressive disease.
Duvelisib plus venetoclax showed a manageable safety profile, with the most common AEs including cytopenias as well as grade 1 or 2 fatigue and diarrhea. Three cases of laboratory TLS were noted. Overall, further exploration of this regimen is warranted in patients with r/r CLL and Richter’s syndrome.
Expanded phase I results for nemtabrutinib
Resistance to BTK inhibitor treatment is often due to mutations at the cysteine-binding residue (C481) on BTK or mutations in PLCγ2 [25, 26]. Nemtabrutinib has been developed as a potent, reversible non-covalent inhibitor of wild-type and ibrutinib-resistant C481S-mutated BTK [27]. The phase I/II BELLWAVE-001 study was designed to investigate nemtabrutinib in patients with relapsed or refractory hematologic malignancies. In patients with CLL/SLL, initial results from the dose-expansion phase revealed promising activity and a manageable AE profile [28].
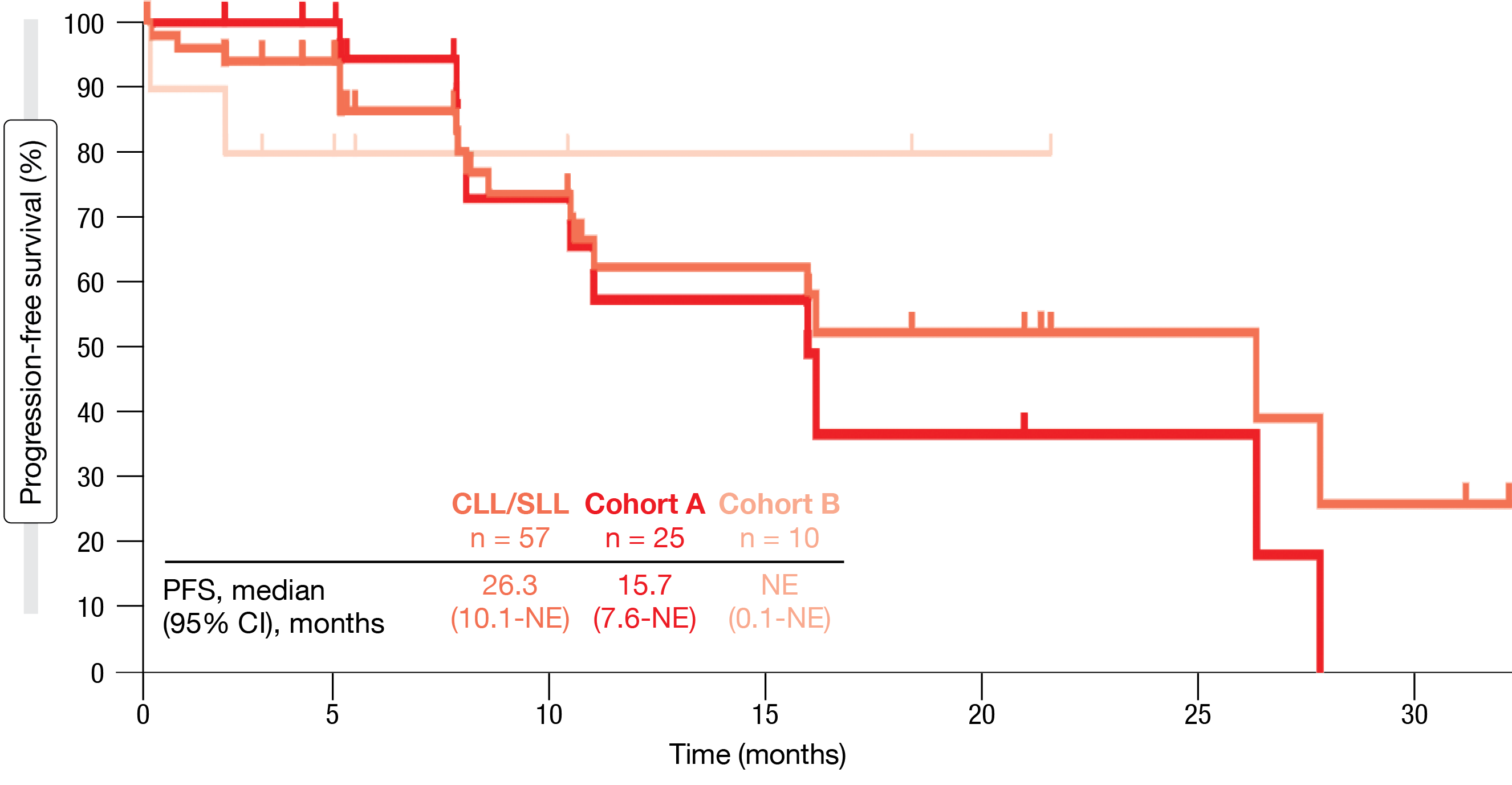
According to the analysis presented at ASH 2022, nemtabrutinib at the recommended phase II dose of 65 mg QD continued to show promising and durable antitumor efficacy [29]. The ORR was 56 % in the overall cohort (n = 57) and 58 % in the BTK- and Bcl-2-pretreated individuals (n = 24), respectively. Patients with C481S mutation responded in 60 % and those without C481S mutation in 40 %. Median PFS was 26.3 months in the overall group, 15.7 months in the C481S-mutated cohort (Cohort A) and had not been reached in the group without C481S mutation (Cohort B) (Figure 4). Patients who were pretreated with BTK and Bcl-2 inhibitors experienced median PFS of 10.1 months and median duration of response of 8.5 months.
The AE profile of nemtabrutinib was manageable. Treatment-related AEs mainly comprised dysgeusia, decreased neutrophil counts, fatigue, decreased platelet counts and nausea. Among AEs of special interest, hypertension was most commonly observed (30 %), followed by arthralgia (20 %), maculopapular rash, and pneumonia (14 % each). In their summary, the authors concluded that nemtabrutinib warrants further investigation in the subgroup of patients with CLL/SLL previously treated with BTK and Bcl-2 inhibitors.
Figure 4: Progression-free survival with nemtabrutinib in the entire group as well as patients with C481S mutation (Cohort A) and without C481S mutation (Cohort B)
REFERENCES
- Sharman JP et al., Understanding ibrutinib treatment discontinuation patterns for chronic lymphocytic leukemia. Blood 2017; 130(suppl 1): 4060
- Mato AR et al., Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018; 103(5): 864-879
- Munir T et al., Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 2019; 94(12): 1353-1363
- Ghia P et al., Ibrutinib treatment in the first-line setting for patients with chronic lymphocytic leukemia: up to 7 years of follow-up in the RESONATE-2 study. EHA 2021, abstract EP636
- Tam CS et al., Clinical pharmacology and PK/PD translation of the second-generation Bruton‘s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol 2021; 14(11): 1329-1344
- Ou YC et al., Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leukemia & Lymphoma 2021; 62(11): 2612-2624
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 2022; 23(8):1031-1043
- Brown JR et al., Zanubrutinib demonstrates superior progression-free survival compared with ibrutinib for treatment of relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: Results from final analysis of ALPINE randomized phase 3 study. ASH 2022, abstract LBA-6
- Allan JN et al., Treatment outcomes after undetectable MRD with first-line ibrutinib plus venetoclax: fixed-duration treatment versus continued ibrutinib with up to 5 years follow-up in the CAPTIVATE study. ASH 2022, abstract 92
- Deng J et al., Bruton‘s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017; 31(10): 2075-2084
- Sharman JP et al., Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022; 36(4):1171-1175
- Davids MS et al., Contribution of obinutuzumab to acalabrutinib therapy in patients with treatment-naïve chronic lymphcytic leukemia: analysis of survival outcomes by genomic features. ASH 2022, 1815
- Davids MS et al., Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study. Lancet Oncol 2021; 22(10): 1391-1402
- Ryan CE et al., Updated results from a multicenter, phase 2 study of acalabrutinib, venetoclax, obinutuzumab (AVO) in a population of previously untreated patients with CLL enriched for high-risk disease. ASH 2022, abstract 344
- Huber H et al., Final analysis of the prospective multicenter phase 2 CLL2-GIVe trial of the German CLL study group – obinutuzumab (GA101), ibrutinib, and venetoclax in untreated CLL with 17p deletion/TP53 mutation. ASH 2022, abstract 343
- Ailawadhi S et al., First-in-human study of lisaftoclax (APG-2575), a novel BCL-2 inhibitor, in patients with relapsed/refractory CLL and other hematologic malignancies. J Clin Oncol 39, 2021 (suppl 15; abstr 7502)
- Davids MS et al., Lisaftoclax safety and activity as monotherapy or combined with acalabrutinib or rituximab in patients with treatment-naïve, relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: initial data from a phase 2 global study. ASH 2022, abstract 964
- Kater A et al., Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. N Engl J Med Evid 2022; 1(7)
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Cheah CY et al., A phase 1 study with the novel B-cell lymphoma 2 (Bcl-2) inhibitor BGB-11417 as monotherapy or in combination with zanubrutinib in patients with CLL/SLL: preliminary data. ASH 2022, abstract 962
- Roberts AW et al., Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia.
N Engl J Med 2016; 374(4): 311-322 - Iannello A et al., Synergistic efficacy of the dual PI3K-δ/γ inhibitor duvelisib with the Bcl-2 inhibitor venetoclax in Richter syndrome PDX models. Blood 2021; 137(24): 3378-3389
- Crombie JL et al., Updated results from a phase I/II study of duvelisib and venetoclax in patients with relapsed or refractory CLL/SLL or Richter’ syndrome. ASH 2020, abstract 3141
- Ryan CE et al., Updated results from a phase I/II study of duvelisib and venetoclax in patients with relapsed or refractory chronic lymphocytic leukemia or Richter’s syndrome. ASH 2022, abstract 4433
- Woyach J et al., Resistance mechanisms for the Bruton‘s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370(24): 2286-2294
- Jones D et al., PLCG2 C2 domain mutations co-occur with BTK and PLCG2 resistance mutations in chronic lymphocytic leukemia undergoing ibrutinib treatment. Leukemia 2017; 31(7): 1645-1647
- Woyach J et al., Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood 2019; 134: 4298
- Woyach JA et al., Preliminary efficacy and safety of MK-1026, a non-covalent inhibitor of wild-typ and C481S mutated Bruton tyrosine kinase, in B-cell malignancies: a phase 2 dose expansion study. Blood 2021; 138: 392
- Woyach J et al., Efficacy and safety of nemtabrutinib, a wild-type and C481S-mutated Bruton tyrosine kinase inhibitor for B-cell malignancies: updated analysis of the open-label, dose-expansion, phase I/II BELLWAVE-001 study. ASH 2022, abstract 3114
© 2023 Springer-Verlag GmbH, Impressum
More posts
Clinical findings with sundry targets in various B-cell malignancies
Clinical findings with sundry targets in various B-cell malignancies AUGMENT: 5-year r
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches Advanced-stage follicular
New approaches in relapsed and refractory DLBCL
New approaches in relapsed and refractory DLBCL Polatuzumab vedotin plus R-ICE Approxi
Chronic lymphocytic leukemia: moving towards new horizons
Chronic lymphocytic leukemia: moving towards new horizons Final analysis of ALPINE: za
Further steps to improve efficacy and safety in acute myeloid leukemia
Further steps to improve efficacy and safety in acute myeloid leukemia Long-term follo
Active monotherapies and combinations in mantle cell lymphoma
Active monotherapies and combinations in mantle cell lymphoma First-line triple combin