Early-stage NSCLC: current insights into perioperative strategies
Overall survival superiority in ADAURA
The randomized phase III ADAURA study was conducted in response to the unmet need of improving 5-year overall survival (OS) rates in patients with completely resected EGFR-mutated, stage IB-IIIA non-small cell lung cancer (NSCLC), which are estimated to range between 45 % and 85 % [1-3]. In ADAURA, 682 patients after complete resection of stage IB, II, or IIIA NSCLC with or without adjuvant chemotherapy received either osimertinib 80 mg OD or placebo for 3 years. Adjuvant osimertinib was shown to improve disease-free survival (DFS) vs. placebo in a statistically significant and clinically meaningful manner in both the primary (i.e., stages II-IIIA) and the overall population (stages IB-IIIA) [4-7]. At ASCO 2023, Herbst et al. presented the OS results from the trial [8].
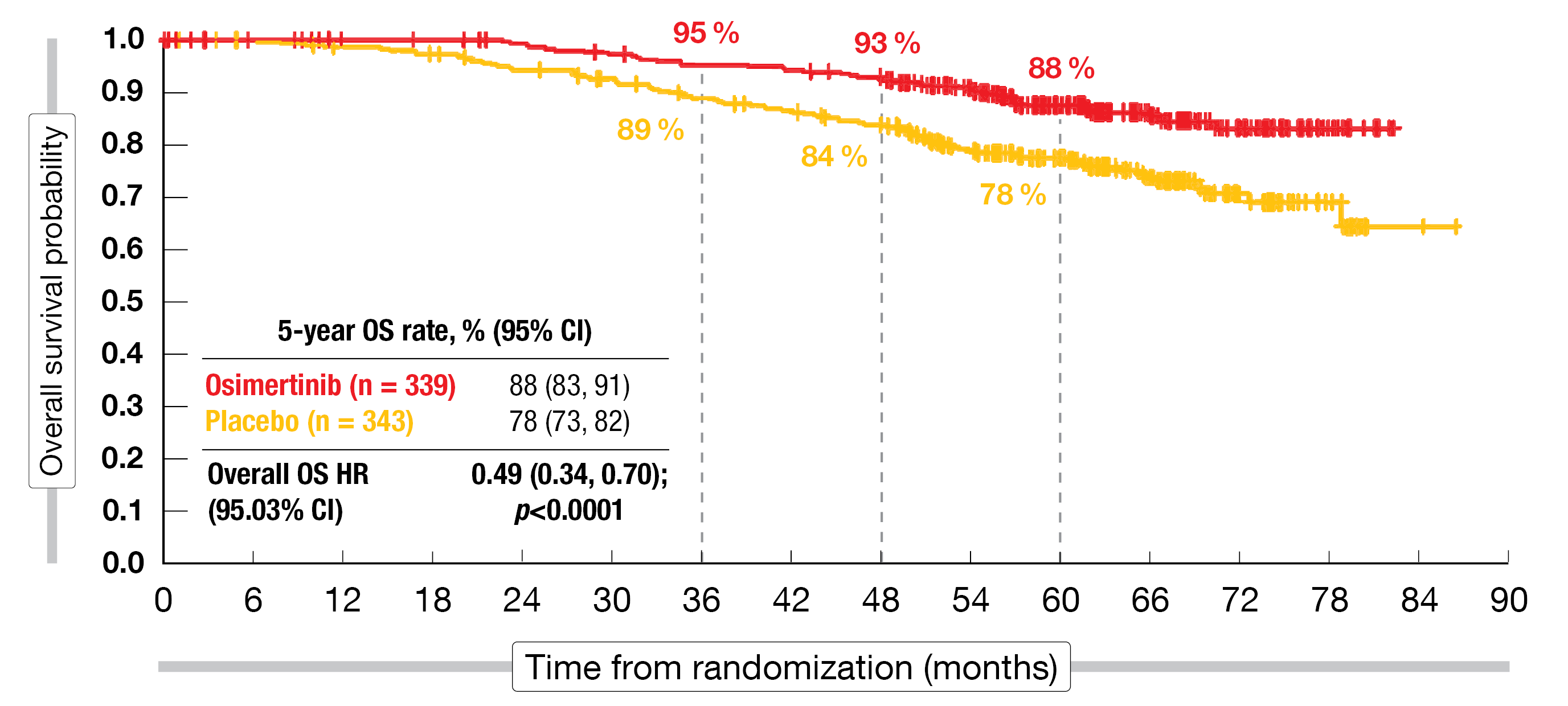
ADAURA is the first global phase III study to demonstrate a statistically significant and clinically meaningful OS benefit with targeted treatment in this patient group. Previously, no phase III trial assessing EGFR tyrosine kinase inhibition (TKI) had demonstrated translation of a DFS advantage into a significant OS benefit [9]. In the primary population, the 5-year OS rates were 85 % vs. 73 % (HR, 0.49; p = 0.0004), and in the overall population, 88 % vs. 78 % (HR, 0.49; p < 0.0001; Figure 1). Both of these analyses resulted in risk reductions of 51 % compared to placebo.
The OS advantage was generally consistent across subgroups. Mortality reductions in stages IB, II and IIIA were 56 %, 37 % and 63 %, respectively. Both patients with and without adjuvant chemotherapy derived risk reductions of more than 50 % when treated with osimertinib. Subsequent anti-cancer therapies were administered in 22 % vs. 54 % of patients treated with osimertinib and placebo, respectively, with EGFR TKIs being the most common strategy across both arms. The safety findings observed during the extended follow-up were consistent with the primary ADAURA analysis [4]. No treatment-related deaths occurred in either arm.
In their conclusion, the authors noted that these results reinforce adjuvant osimertinib as the standard of care for patients with resected EGFR-mutant, stage IB-IIIA NSCLC. Ongoing osimertinib studies in the setting of resectable EGFR-mutated NSCLC include the phase III NeoADAURA study (NCT04351555), the phase III ADAURA2 study (NCT05120349) and the phase II TARGET trial (NCT05526755).
Figure 1: Overall survival advantage of adjuvant osimertinib compared to placebo in the ADAURA trial
No MPR improvement with neoadjuvant osimertinib
A multicenter phase II trial was initiated to assess the neoadjuvant use of osimertinib in patients with resectable stage I-IIIA, EGFR-mutated NSCLC based on the assumption that targeted therapy might improve MPR rates, thus leading to survival prolongation. Twenty-seven individuals received neoadjuvant osimertinib for 1–2 months prior to surgical resection.
With 15 % of patients achieving major pathological responses (MPR), the study did not meet its primary endpoint, which was defined as an MPR rate of 50 % [10]. Pathological responses occurred in 48 %, and lymph node downstaging resulted in 44 %. None of the patients achieved pathological complete response (pCR). The R0 surgical resection rate was 89 %. No surgical complications occurred; serious adverse events (AEs) and perioperative complications were in line with the predicted rates in this population. Median DFS was 32.4 months.
The scientists used exome sequencing, single-cell RNA sequencing and multiplex immunohistochemistry to explore molecular mechanisms of disease persistence, which is an issue in both early-stage and advanced EGFR-mutated NSCLC. According to these findings, co-occurring loss-of-function mutations in RBM10 might limit patient responses. The addition of the Bcl-xL inhibitor navitoclax to osimertinib was shown to induce apoptosis in RBM10-deficient preclinical models [11], although this observation needs to be confirmed in clinical trials. Moreover, upregulation of the YAP pathway appeared to drive tumor cell survival, which might provide another target for combination therapies to eliminate residual disease.
KEYNOTE-671: perioperative immunotherapy
Although immune checkpoint inhibition has proven beneficial in phase III trials when administered before or after resection of early-stage NSCLC, recurrence is still common [12-14]. Therefore, it was hypothesized that a perioperative approach including both neoadjuvant and adjuvant PD-(L)1 inhibition might be superior to either approach alone. The randomized, double-blind, phase III KEYNOTE-671 trial assessed neoadjuvant pembrolizumab 200 mg Q3W plus chemotherapy (i.e., cisplatin plus gemcitabine or pemetrexed) vs. placebo plus chemotherapy for up to 4 cycles in patients with resectable stage II, IIIA, or IIIB (N2) NSCLC. After surgery, the patients allocated to the experimental arm went on to receive pembrolizumab 200 Q3W for up to 13 cycles, while placebo was administered to those in the control arm. Event-free survival (EFS) per investigator review and OS were defined as the dual primary endpoints.
The analysis presented at ASCO 2023 after a median follow-up of 25.2 months included 397 and 400 individuals randomized to the experimental and control arms, respectively [15]. Among these, 325 and 317, respectively, had received in-study surgery. R0 resection was performed in 92.0 % and 84.2 %, respectively. Lobectomy represented the most commonly used surgical procedure (78.8 % and 75.1 %, respectively). The study population contained patients with known EGFR mutations (3.5 % and 4.8 %, respectively) and ALK translocations (3.0 % and 2.3 %, respectively).
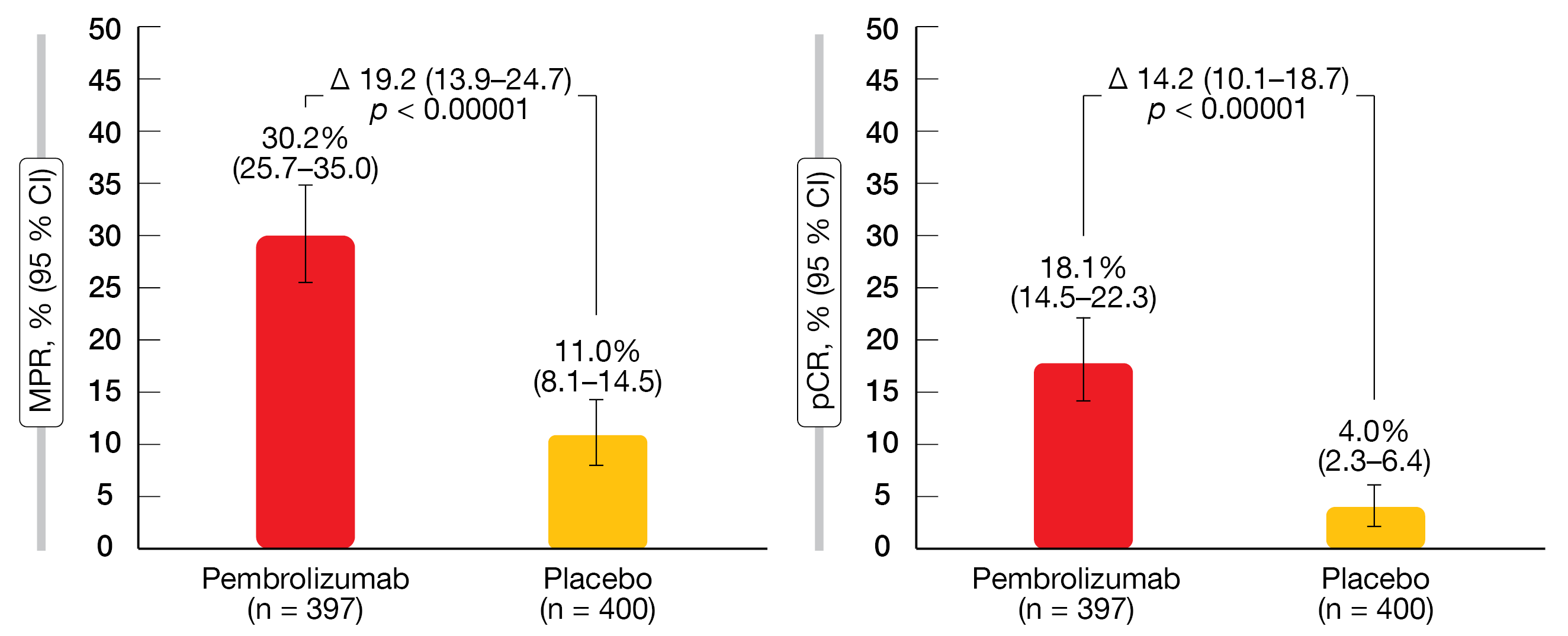
Indeed, the perioperative strategy, as compared to neoadjuvant chemotherapy and surgery alone, provided statistically significant and clinically meaningful EFS improvement (median EFS, not reached vs. 17.0 months; HR, 0.58; p < 0.00001). At 12 months, the EFS rates were 73.2 % vs. 59.9 %, and at 24 months, 62.4 % vs. 40.6 %. The EFS benefit was generally consistent across all patient and disease characteristics analyzed, which included histology, disease stage and PD-L1 levels. Patients in the pembrolizumab arm experienced significantly higher rates for both MPR and pCR (p < 0.00001 for both; Figure 2). An exploratory analysis showed that EFS benefits were more likely in the groups that achieved MPR and pCR across the study arms. However, pembrolizumab-treated patients generally fared better than control patients.
Regarding OS, the significance boundary had not been crossed yet, although the curves have started to separate (median OS, not reached vs. 45.5 months; HR, 0.73). The AE profile observed in KEYNOTE-671 was as expected based on the known profiles of the individual treatment components. Grade 3–5 immune-related AEs and infusion reactions occurred in 5.8 % vs. 1.5 % of patients, with one pneumonitis-related fatality in the pembrolizumab-treated group (0.3 %). Overall, the results from the KEYNOTE-671 trial support perioperative pembrolizumab as a promising new treatment option for patients with resectable stage II, IIIA, or IIIB (N2) NSCLC.
Figure 2: Pathological responses achieved with perioperative pembrolizumab plus chemotherapy vs. neoadjuvant chemotherapy plus surgery alone
NEOpredict: nivolumab plus relatlimab
Given the demand for novel agents in the curative setting, the randomized, multicenter phase II NEOpredict-Lung study investigated the feasibility, safety and early efficacy of the combined preoperative treatment with nivolumab and the anti-LAG-3 antibody relatlimab. Patients with stage IB, II and selected IIIA NSCLC were randomized to either nivolumab 240 mg plus relatlimab 80 mg on days 1 and 15 (n = 30) or nivolumab alone (n = 30) followed by surgery and standard-of-care adjuvant treatment. Meanwhile, a third study arm has been added to investigate nivolumab plus relatlimab at an increased dose. The feasibility of curatively intended surgery within 43 days of treatment was the primary endpoint.
All patients in both treatment arms met the primary objective [16]. Curative resection was obtained in 98.3 % across the arms. Most patients underwent lobectomy (24 and 23 in the combination and nivolumab monotherapy arms, respectively). Video-assisted thoracoscopic surgery was possible in 63.3 % vs. 60 %; two vs. three patients had to undergo conversion to thoracotomy. Perioperative complications occurred in 26.6 % vs. 33.3 %, which was within the expected range. One patient each (3 %) required intraoperative conversion due to bleeding. Surgical revision was necessary in one individual in the experimental arm (3 %) due to middle lobe torsion and in two patients in the control arm (7 %) because of empyema and prolonged air leak. In the nivolumab-only arm, there was one case of pulmonary embolism. No patient died within 30 days.
Regarding histopathological response, the regimens gave rise to similar pCR (16.7 % vs. 13.3 %) and MPR (30 % vs. 27 %) rates. Deeper responses were observed in patients with higher PD-L1 expression. Adjuvant therapy was indicated in 14 patients in each group and could be safely administered in all cases. Across both groups, the 12-month OS rate was 96 %, and the 12-month DFS rate was 91 %. Grade ≥ 3 immunotherapy-related AEs occurred in one patient in the combination arm and three in the monotherapy arm.
In their conclusion, the authors noted that perioperative course, morbidity and mortality rates observed in this study were comparable to those observed with other neoadjuvant regimens. Both minimally invasive and open surgical techniques were safe, and there appeared to be no increased risk of complications in bronchial and/or vascular sleeve resections. Comprehensive correlative studies and biomarker analyses are ongoing.
Toripalimab plus chemotherapy
More than 400 patients with resectable stage II and III NSCLC have been enrolled into the randomized, double-blind, phase III NeoTORCH trial that is investigating the perioperative use of the anti–PD-1 monoclonal antibody toripalimab. In the experimental arm, toripalimab 240 mg Q3W is being administered in addition to neoadjuvant chemotherapy for 3 cycles; after surgery, patients receive 1 adjuvant cycle of the same regimen and go on to maintenance treatment with toripalimab Q3W for up to 13 cycles. In the control arm, placebo is used instead of the PD-1 inhibitor. EFS according to investigator in stages III and II/III as well as MPR according to blinded independent pathologic review in stages III and II/III are defined as the primary objective. At ASCO 2023, Lu et al. presented the results of the interim EFS analysis that related to 202 patients in each study arm [17].
In the combination and chemotherapy-alone arms, 87.1 % and 91.6 % of patients, respectively, completed 3 neoadjuvant cycles. Surgery was performed in 82.2 % vs. 73.3 %. Adjuvant treatment was administered in 71.3 % and 64.9 %, respectively, and similar percentages of patients received maintenance. Thirteen cycles of maintenance treatment were completed by 43.6 % and 32.7 %, respectively. R0 resection resulted in 95.8 % vs. 92.6 %.
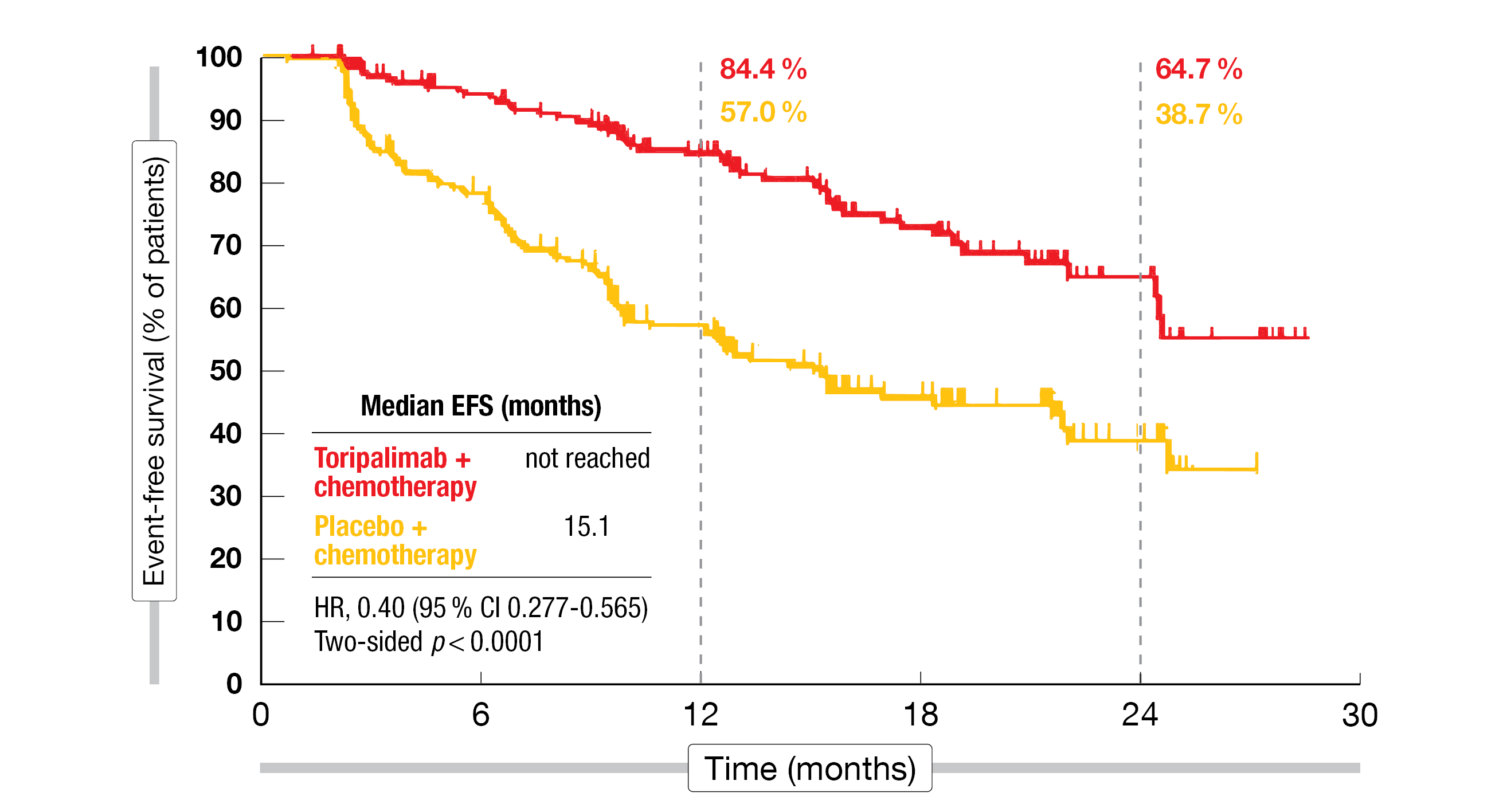
With respect to the primary endpoint of EFS by investigator in patients with stage III disease, the analysis revealed superiority of the immunotherapy-based approach, translating into a 60 % risk reduction (not reached vs. 15.1 months; HR, 0.40; two-sided p < 0.0001; Figure 3). The EFS rates at 24 months were 64.7 % vs. 38.7 %. Improvement in EFS was consistent across all key subgroups including the PD-L1 cohorts, with HRs of 0.59, 0.31 and 0.31 for the PD-L1 < 1 %, 1-49 % and ≥ 50 % subgroups, respectively. Benefits were seen in both non-squamous (HR, 0.54) and squamous (HR, 0.35) subtypes.
Considerable differences favoring the toripalimab combination were observed regarding the pCR rate (24.8 % vs. 1 %; p < 0.0001) and the MPR rate (48.5 % vs. 8.4 %; p < 0.0001). Patients who had achieved MPR or pCR showed longer EFS with both treatments than those who had not. The OS analysis revealed a trend in favor of the combined strategy (2-year OS rates, 81.2 % vs. 74.3 %; HR, 0.62). Toripalimab plus chemotherapy was well tolerated. Treatment-emergent AEs leading to interruption of the PD-1 inhibitor were reported in 28.2 % (vs. interruption of placebo in 14.4 %), and discontinuation was necessary in 9.4 % (vs. 7.4 %). One fatal treatment-related event occurred in the experimental arm (0.5 % vs. 0.0 %). Grade ≥ 3 immune-related AEs were seen in 11.9 % vs. 3.0 %, and any-grade infusion-related reactions in 3.5 % vs. 6.4 %. Surgery-related postoperative AEs necessitated interruption of toripalimab in 6.6 % (vs. interruption of placebo in 1.4 %) and discontinuation in 1.8 % (vs. 2.7 %). As the authors emphasized, the results from NeoTORCH are in keeping with those from other studies suggesting that perioperative immunotherapy plus chemotherapy should be a standard of care for patients with stage III NSCLC.
Figure 3: NeoTORCH: event-free survival in the intent-to-treat stage III population according to investigator
CheckMate 816: 3-year findings according to surgery status
Neoadjuvant nivolumab in addition to chemotherapy for 3 cycles is being assessed in patients with resectable stage IB-IIIA NSCLC included in the phase III CheckMate 816 trial. Compared to neoadjuvant chemotherapy only, the combination significantly improved EFS and pCR, with continued EFS benefit observed at 3 years [12, 18]. At ASCO 2023, Spicer et al. reported an exploratory analysis of the 3-year outcomes in patients who underwent definitive surgery (149 and 135 for nivolumab plus chemotherapy and chemotherapy only, respectively) versus those who did not (30 and 44, respectively) [19].
In addition to the proportion of patients who had surgery being numerically higher in the experimental arm compared to the control arm, the combination gave rise to improved outcomes independent of surgery status, although patients without definitive surgery only showed numerical benefits. In the operated group, median EFS had not been reached with nivolumab plus chemotherapy and was 31.8 months with chemotherapy alone (HR, 0.67). In the cohort without surgery, this was 6.7 vs. 4.1 months (HR, 0.75). Time to distant metastasis was prolonged with the addition of nivolumab both in the resected cohort (not reached vs. 46.8 months; HR, 0.55) and the non-resected group (24.8 vs. 15.6 months; HR, 0.63). EFS2, which was defined as the time from randomization to objectively documented progression after the next line of therapy or to death from any cause, again favored the immunotherapy-based regimen both in the cohort with definitive surgery (36-month rates, 80 % vs. 69 %) and the one without (33 % vs. 24 %). Patients who could not undergo tumor resection mostly received subsequent chemotherapy and/or radiotherapy.
Neither the incidence nor the severity of AEs was increased by the addition of nivolumab to chemotherapy regardless of surgery status. In patients without definitive surgery, grade 3-4 treatment-related AEs were less common with the combination than with chemotherapy alone (26 % vs. 46 %). The authors noted that these long-term results from CheckMate 816 continue to corroborate neoadjuvant nivolumab plus chemotherapy as a standard treatment option for patients with resectable NSCLC.
Pembrolizumab after adjuvant chemotherapy
Pembrolizumab 200 mg Q3W for ≤ 18 administrations was tested against placebo after complete resection of stage IB-IIIA NSCLC in the randomized, triple-blind, phase III PEARLS/KEYNOTE-091 study. Adjuvant chemotherapy for up to 4 cycles was administered prior to pembrolizumab as indicated. In the overall population (n = 1,177), DFS was significantly improved in the experimental arm (53.6 vs. 42.0 months; HR, 0.76; p = 0.0014) [20]. Oselin et al. presented the findings in the group that had received 1–4 cycles of adjuvant chemotherapy per protocol; these constituted 86 % (n = 1,010) of the randomized patients [21].
In this group, consistent with the results in the ITT population, pembrolizumab gave rise to improved DFS compared to placebo, resulting in a 27 % risk reduction (58.7 vs. 34.9 months; HR, 0.73). At 18 months, 73.8 % vs. 63.1 % of patients were disease-free. Similar risk reductions were obtained in patients after 1 or 2 (HR, 0.59) and 3 chemotherapy cycles (HR, 0.56). Patients with EGFR-mutant disease benefited greatly from pembrolizumab treatment (HR, 0.39). Across the stages, stage IB was associated with the greatest risk reduction (HR, 0.54).
Results from IMpower010 by KRAS mutational status
Atezolizumab after adjuvant chemotherapy for completely resected stage IB-IIIA NSCLC was compared with best supportive care (BSC) in the randomized, open-label, phase III IMpower010 trial that met its primary DFS endpoint [13]. As KRAS mutations are prevalent in metastatic NSCLC and may be a poor prognostic factor [22, 23], Reck et al. assessed baseline characteristics and DFS outcomes in IMpower010 according to KRAS mutational status [24].
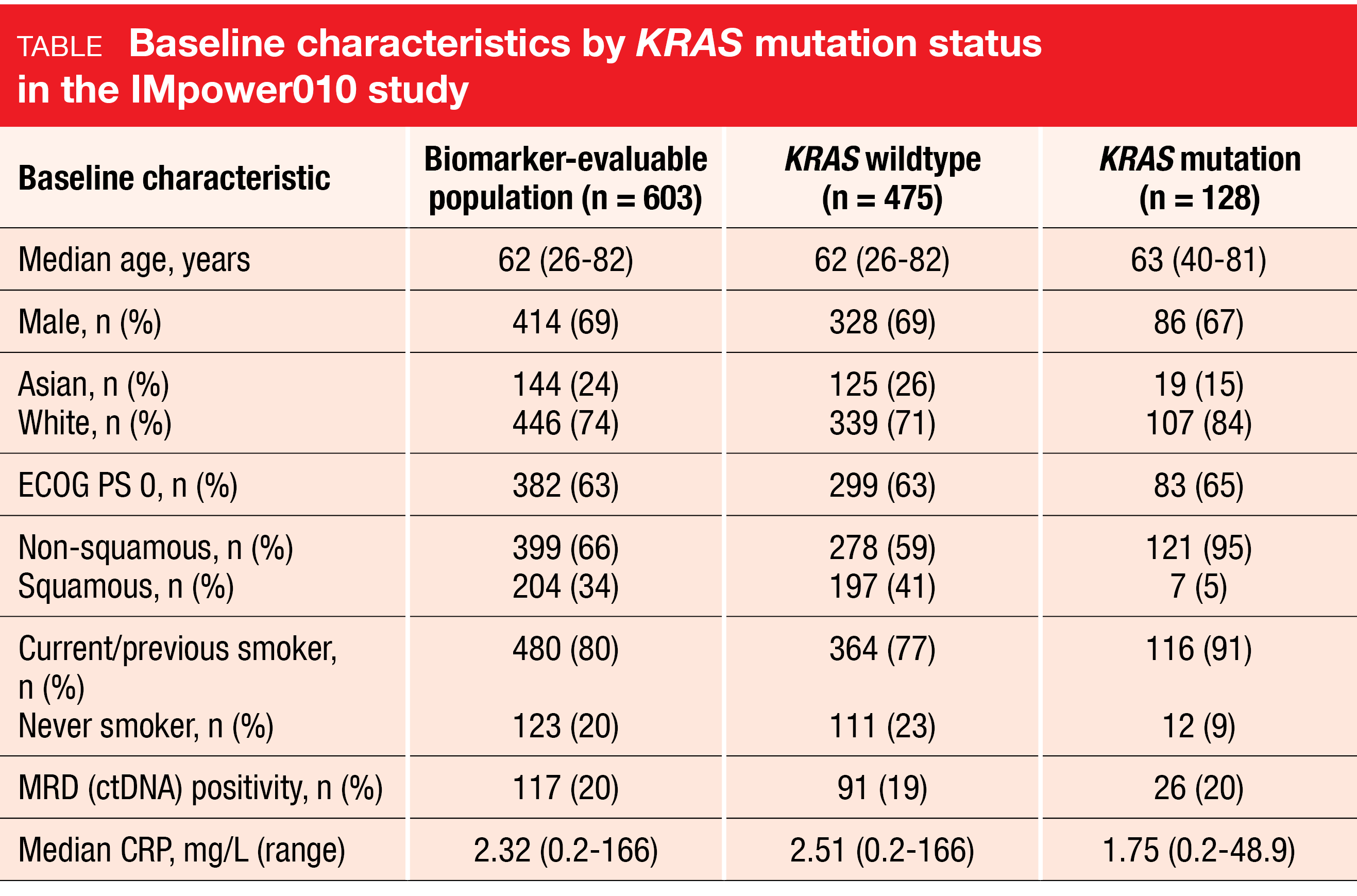
The prevalence of KRAS mutations in this early-stage setting was similar to that reported for metastatic NSCLC [23]. In the biomarker-evaluable population according to whole exome sequencing (n = 603), KRAS mutations were found in 21 % (n = 128). The mutation rates were higher in white vs. Asian patients and in non-squamous vs. squamous tumors (Table). Almost all KRAS mutation carriers were current or previous smokers. In the biomarker-evaluable group with stage II-IIIA disease (n = 536), atezolizumab improved DFS vs. BSC irrespective of KRAS mutational status. Patients with KRAS wildtype derived a 26 % risk reduction (median DFS, 42.3 vs. 31.4 months; HR, 0.74), while this was 44 % in those with KRAS mutation (not reached vs. 25.2 months; HR, 0.56).
Moreover, PD-L1 positivity did not affect the outcomes in the KRAS-mutated subgroup with stage II-IIIA disease. PD-L1–positive samples were present in 59 % in the KRAS-mutated subgroup and in 53 % in the KRAS wildtype subgroup. Atezolizumab treatment improved DFS compared to BSC for both PD-L1–negative (median DFS, not reached vs. 31.6 months) and PD-L1–positive (not reached vs. 21.7 months) patients. However, the authors cautioned that this analysis is limited by the small sample sizes.
REFERENCES
- Kelly K et al., Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol 2015; 33(34): 4007-4014
- Zhong WZ et al., Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: Final overall survival analysis of CTONG1104 phase III Trial. J Clin Oncol 2021; 39(7): 713-722
- Aokage K et al., Prognostic influence of epidermal growth factor receptor mutation and radiological ground glass appearance in patients with early-stage lung adenocarcinoma. Lung Cancer 2021; 160: 8-16
- Wu YL et al., Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020; 383(18): 1711-1723
- Herbst et al., Osimertinib as adjuvant therapy in patients with stage IB–IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 2020; 38(Suppl 18): LBA5
- Herbst et al., Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: Updated results from the phase III randomized ADAURA trial. J Clin Oncol 2023; 41(10): 1830-1840
- Tsuboi et al., Osimertinib as adjuvant therapy in patients with resected EGFR-mutated stage IB-IIIA non-small cell lung cancer: Updated results from ADAURA. Ann Oncol 2022; 33(Suppl 7): S808-S869
- Herbst R et al., Overall survival analysis from the ADAURA trial of adjuvant osimertinib in patients with resected EGFR-mutated stage IB-IIIA non-small cell lung cancer. J Clin Oncol 41, 2023 (suppl 17; abstr LBA3)
- de Scordilli M et al., Targeted therapy and immunotherapy in early-stage non-small cell lung cancer: Current evidence and ongoing trials. Int J Mol Sci 2022; 23(13): 7222
- Aredo JV et al., Phase II trial of neoadjuvant osimertinib for surgically resectable EGFR-mutated non-small cell lung cancer. J Clin Oncol 2023; 41 (suppl 16; abstr 8508)
- Nanjo S et al., Deficiency of the splicing factor RBM10 limits EGFR inhibitor response in EGFR-mutant lung cancer. J Clin Invest 2022; 132(13): e145099
- Forde PM et al., Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386(21): 1973-1985
- Felip E et al., Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021; 398(10308): 1344-1357
- O’Brien M et al., Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022; 23(10): 1274-1286
- Wakelee H et al., KEYNOTE-671: randomized, double-blind, phase 3 study of pembrolizumab or placebo plus platinum-based chemotherapy followed by resection and pembrolizumab or placebo for early-stage NSCLC.
J Clin Oncol 41, 2023 (suppl 17; abstr LBA100) - Aigner C et al., Surgical outcomes of patients with resectable NSCLC receiving neoadjuvant immunotherapy with nivolumab plus relatlimab or nivolumab. J Clin Oncol 41, 2023 (suppl 16; abstr 8500)
- Lu S et al., Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer: interim event-free survival analysis of the phase III Neotorch study. J Clin Oncol 41, 2023 (suppl 16; abstr 8501)
- Forde PM et al., Neoadjuvant nivolumab + platinum-doublet chemotherapy for resectable NSCLC: 3-year update from CheckMate 816. ELCC 2023, poster 840
- Spicer J et al., Clinical outcomes with neoadjuvant nivolumab plus chemotherapy vs chemotherapy by definitive surgery in patients with resectable NSCLC: 3-year results from the phase 3 CheckMate 816 trial. J Clin Oncol 41, 2023 (suppl 16; abstr 8521)
- Paz-Ares L et al., Pembrolizumab versus placebo for early-stage non-small cell lung cancer following complete resection and adjuvant chemotherapy when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study. Ann Oncol 2022; 33(4): P451-453
- Oselin K et al., Pembrolizumab vs. placebo for early-stage non-small cell lung cancer after resection and adjuvant therapy: subgroup analysis of patients who received adjuvant chemotherapy in the phase 3 PEARLS/KEYNOTE-091 study. J Clin Oncol 41, 2023 (suppl 16; abstr 8520)
- El Osta BE et al., Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: The Lung Cancer Mutation Consortium experience. J Thorac Oncol 2019; 14(5): 876-889
- Yu HA et al., Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J Thorac Oncol 2015; 10(3): 431-437
- Reck M et al., IMpower010: exploratory analysis of disease-free survival by KRAS status in patients with stage II-IIIA NSCLC treated with adjuvant atezolizumab vs best supportive care. J Clin Oncol 41, 2023 (suppl 16; abstr 8522)
© 2023 Springer-Verlag GmbH, Impressum
More posts
Targeted approaches in advanced disease
Targeted approaches in advanced disease Anti-EGFR agents plus inserted chemotherapy Ac
Small-cell lung cancer: novel agents & biomarkers
Small-cell lung cancer: novel agents & biomarkers Atezolizumab plus talazoparib as
Early-stage NSCLC: current insights into perioperative strategies
Early-stage NSCLC: current insights into perioperative strategies Overall survival sup
Preface – ASCO Lung Cancer 2023
Preface – ASCO Lung Cancer 2023 © private – Manali I. Patel, MD MPH MS, Associate Pro