Small-cell lung cancer: novel agents & biomarkers
Atezolizumab plus talazoparib as maintenance
As is known, patients with extensive-stage small-cell lung cancer (ES-SCLC) tend to respond well to systemic induction therapy but frequently experience rapid disease progression. Subsequent treatment success remains a challenge; therefore, improving outcomes in the first line appears critical. Poly (ADP-ribose) polymerase (PARP) 1 has emerged as a potential therapeutic target in neuroendocrine tumors such as SCLC. Also, expression of the Schlafen family member 11 (SLFN11) protein was shown to predict improved overall survival (OS) and progression-free survival (PFS) with the PARP inhibitor veliparib plus temozolomide in SCLC patients [1].
The phase II SWOG S1929 study evaluated maintenance with atezolizumab plus the PARP inhibitor talazoparib in patients with SLFN11-positive ES-SCLC. After 4 cycles of induction chemotherapy and atezolizumab, patients who had SLFN11-positive and non-progressive disease were randomized to either the combination or atezolizumab alone. PFS was defined as the primary objective. According to the results reported at ASCO 2023 by Abdel Karim et al., central SLFN11 testing of archival tissue using immunohistochemistry revealed positivity in 79 % [2]. The analysis included 54 and 52 patients randomized into the combination and monotherapy arms, respectively. SWOG S1929 met its primary endpoint. Atezolizumab plus talazoparib improved PFS compared to atezolizumab alone, resulting in a 30 % risk reduction (median PFS, 4.2 vs. 2.8 months; HR, 0.70; p = 0.056). Patients with prior thoracic radiotherapy and brain metastases represented the groups deriving the most pronounced PFS benefits, with HRs of 0.54 and 0.42, respectively. The response rates after maintenance did not differ across the arms (12 % vs. 16 %). Disease control was achieved in 59 % vs. 69 %.
As expected, the addition of the PARP inhibitor increased hematologic toxicity, which was mainly reflected by higher rates of grade 3 anemia (37 % vs. 2 %). Grade 3 decreases in platelet counts occurred in 17 % vs. 0 %. With respect to non-hematological toxicity, which mainly included transaminitis and hyperglycemia, grade ≥ 3 treatment-related adverse events (AEs) did not differ across the treatment arms (15 % vs. 13 %). Only three patients overall discontinued treatment due to toxicity.
In their conclusion, the authors noted that the SWOG S1929 study demonstrated the feasibility of conducting biomarker-selected trials in the setting of SCLC, thus paving the way for future evaluation of novel therapies in selected SCLC populations. The association between SLFN11 expression levels and clinical outcomes is currently being explored.
First-in-human results for bispecific T-cell engager
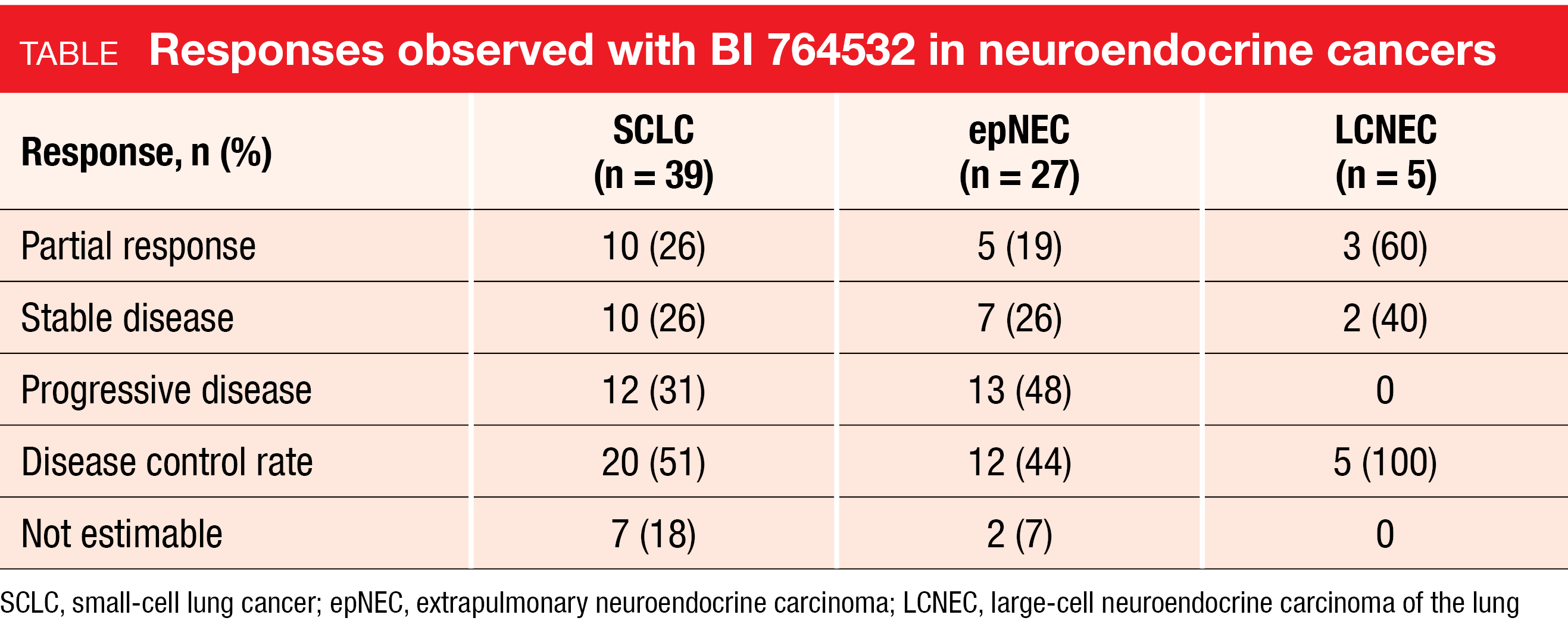
BI 764532 has been developed as a delta-like ligand 3(DLL3)/CD3 IgG-like bispecific T-cell engager with the aim to redirect T cells to tumor sites and induce lysis of DLL3-expressing cancer cells such as SCLC cells. In DLL3-positive cells and xenograft models, BI 764532 has shown potent preclinical anti-tumor activity [3]. Wermke et al. presented the results of the first-in-human dose-escalation trial in patients with DLL3-positive advanced SCLC (n = 57), extrapulmonary neuroendocrine carcinoma (epNEC; n = 41), or large-cell neuroendocrine lung carcinoma (LCNEC; n = 9) at ASCO 2023 [4]. These patients had failed available standard therapies or were ineligible for them. Two thirds had previously received one or two treatment lines, while one third had been treated with ≥ 3 lines. Asymptomatic and stable brain metastases were allowed; therefore, brain lesions were present in 38 % of the study population. Almost half had received prior PD-(L)1 inhibitor therapy. BI 764532 was administered at different dose levels and schedules for a maximum of 36 months.
The compound showed promising efficacy with an overall objective response rate (ORR) of 25 % at doses ≥ 90 µg/kg. Tumor shrinkage was observed across all three entities. For SCLC, epNEC and LCNEC, the ORRs were 26 %, 19 % and 60 % (Table). Responses appeared durable as they were ongoing in 14 out of 18 responders at data cutoff, with a few exceeding already 12 months. Median duration of response had not been reached yet.
Moreover, the AE profile of BI 764532 at clinically efficacious dose levels was acceptable and manageable. Cytokine release syndrome (CRS) occurred as the most common side effect (all grades, 59 %), followed by decreased lymphocyte counts (20 %), dysgeusia (20 %), and asthenia (19 %). Almost all CRS events were graded as 1 or 2, emerged during the initial drug administrations, and were manageable with supportive care, corticosteroids and/or anti-IL-6R antibodies. Treatment-related AEs led to discontinuation in 4 % only. Dose-limiting toxicity events including CRS grade 3-4, infusion-related reaction and central nervous system AEs proved reversible. The maximum tolerated dose had not been reached at the time of the analysis. Further dose optimization is ongoing.
Biomarker analysis of KEYNOTE-604
The phase III KEYNOTE-604 study has shown PFS improvement with first-line pembrolizumab plus etoposide and platinum (EP) chemotherapy vs. EP alone in patients with ES-SCLC [5]. Notably, PFS and OS improvements were observed regardless of the PD-L1 CPS, which indicated that this marker was not predictive of the treatment outcomes. Rudin et al. reported an exploratory biomarker analysis of KEYNOTE-604 at ASCO 2023 that determined the association between efficacy outcomes and tumor mutational burden (TMB), T cell-inflamed gene expression profile (TcellinfGEP), and monocytic myeloid-derived suppressor cells (mMDSC) as well as granulocytic myeloid-derived suppressor cells (gMDSC) as part of the non-gene expression profile signature [6]. Assessments were based on whole exome sequencing of tumor tissue and matched normal DNA and RNA sequencing. The biomarker-evaluable population in the experimental arm included 167 and 159 patients for the TMB and TcellinfGEP analysis, respectively; in the control arm, these were 151 and 157 individuals, respectively.
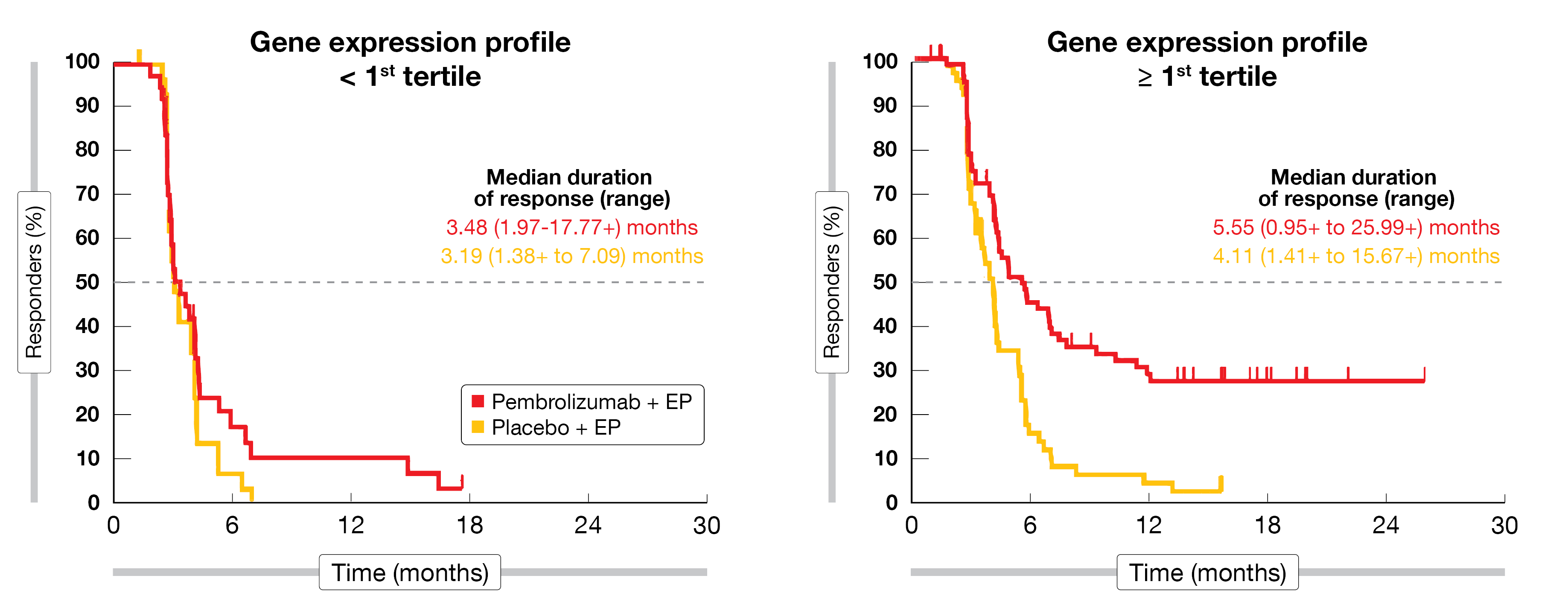
According to the data obtained, TMB was not positively associated with OS or PFS in the pembrolizumab plus EP group. At the same time, there was a statistically significant association between TMB and OS in the control arm, which appears to be a false-positive result based on underperformance of the chemotherapy-only regimen in the group with low TMB. The TcellinfGEP assessment yielded significant associations with OS and PFS not only in the experimental arm but to a similar extent in both treatment groups. Interestingly, patients who experienced longer duration of response tended to have high TcellinfGEP at baseline, with long-term responders being virtually restricted to the pembrolizumab-treated groups (Figure). Low expression of mMDSC and gMDSC was potentially associated with longer PFS and OS in the pembrolizumab-treated patients.
Overall, the authors concluded that the benefit of pembrolizumab plus EP was observed across most of the biomarker subgroups analyzed. The data will need to be interpreted in the context of similar analyses of other trials to understand their role in SCLC.
Figure: Association between duration of response with pembrolizumab plus chemotherapy and T cell-inflamed gene expression profile in KEYNOTE-604
REFERENCES
- Pietanza MC et al., Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J Clin Oncol 2018; 36(23): 2386-2394
- Abdel Karim NF et al., Phase II randomized study of maintenance azolizumab versus atezolizumab + talazoparib in patients with SLFN11 positive extensive stage small cell lung cancer.
J Clin Oncol 41, 2023 (suppl 16; abstr 8504) - Hipp S et al., A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 2020; 26(19): 5258-5268
- Wermke M et al., First-in-human dose-escalation trial of BI 764532, a delta-like ligand 3/CD3 IgG-like T-cell engager, in patients with DLL3-positive small-cell lung cancer and neuroendocrine carcinoma. J Clin Oncol 41, 2023 (suppl 16; abstr 8502)
- Rudin CM et al., Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 Study. J Clin Oncol 2020; 38(21): 2369-2379
- Rudin CM et al., Exploratory biomarker analysis of the phase 3 KEYNOTE-604 study of pembrolizumab plus etoposide for extensive-stage SCLC. J Clin Oncol 41, 2023 (suppl 16; abstr 8503)
© 2023 Springer-Verlag GmbH, Impressum
More posts
Targeted approaches in advanced disease
Targeted approaches in advanced disease Anti-EGFR agents plus inserted chemotherapy Ac
Small-cell lung cancer: novel agents & biomarkers
Small-cell lung cancer: novel agents & biomarkers Atezolizumab plus talazoparib as
Early-stage NSCLC: current insights into perioperative strategies
Early-stage NSCLC: current insights into perioperative strategies Overall survival sup
Preface – ASCO Lung Cancer 2023
Preface – ASCO Lung Cancer 2023 © private – Manali I. Patel, MD MPH MS, Associate Pro