Targeted approaches in advanced disease
Anti-EGFR agents plus inserted chemotherapy
Acquired resistance to EGFR tyrosine kinase inhibitors (TKIs), even including the third-generation agent osimertinib, limits duration of response and survival in treated patients with EGFR-mutant lung tumors. A potential strategy to improve outcomes is the concomitant use of EGFR-targeted agents and platinum doublet chemotherapy [1, 2], although EGFR TKI treatment might attenuate the effect of cytotoxic agents [3-6]. According to the Norton-Simon hypothesis, however, sensitivity to chemotherapy might be increased dependent on timing [7-9]. Therefore, it was hypothesized that chemotherapy inserted after the initial response to EGFR TKI treatment, rather than at the time of progression, might lead to the elimination of emerging resistant clones. Most tumor cells would thus be EGFR-TKI–sensitive when EGFR-targeted treatment is resumed, and survival might be prolonged.
A phase II study has tested first-line treatment with gefitinib 250 mg OD on days 1-56 followed by chemotherapy for 3 courses on days 71, 92 and 113 before gefitinib was resumed from day 134 until disease progression [10]. This strategy appeared to prevent the development of acquired resistance to EGFR TKI treatment, with favorable progression-free survival (PFS) of 19.5 months and overall survival (OS) of 48.0 months.
The open-label, randomized, phase III JCOG1404/WJOG8214L study was conducted to confirm this observation. Patients with non-squamous, stage IIIB/IV or recurrent NSCLC that harbored EGFR exon 19 deletions or L858R mutations were enrolled. In the experimental arm, gefitinib was administered according to the same schedule as in the phase II trial; additionally, three courses of chemotherapy with cisplatin and pemetrexed were inserted on days 71, 92 and 113. Patients in the control arm received gefitinib 250 mg OD from day 1 until progression. The EGFR TKI used in the trial was changed to osimertinib 80 mg OD in October 2018 based on the results of the FLAURA study [11]. Overall, the experimental and control arms included 251 and 250 patients, respectively. Among these, 96 and 97, respectively, received osimertinib, while 155 and 153, respectively, had been treated with gefitinib. Stable CNS metastases were present in 28.3 % of the total population. OS constituted the primary endpoint. Kanda et al. reported the findings at ASCO 2023 [12].
PFS benefit, but no OS advantage
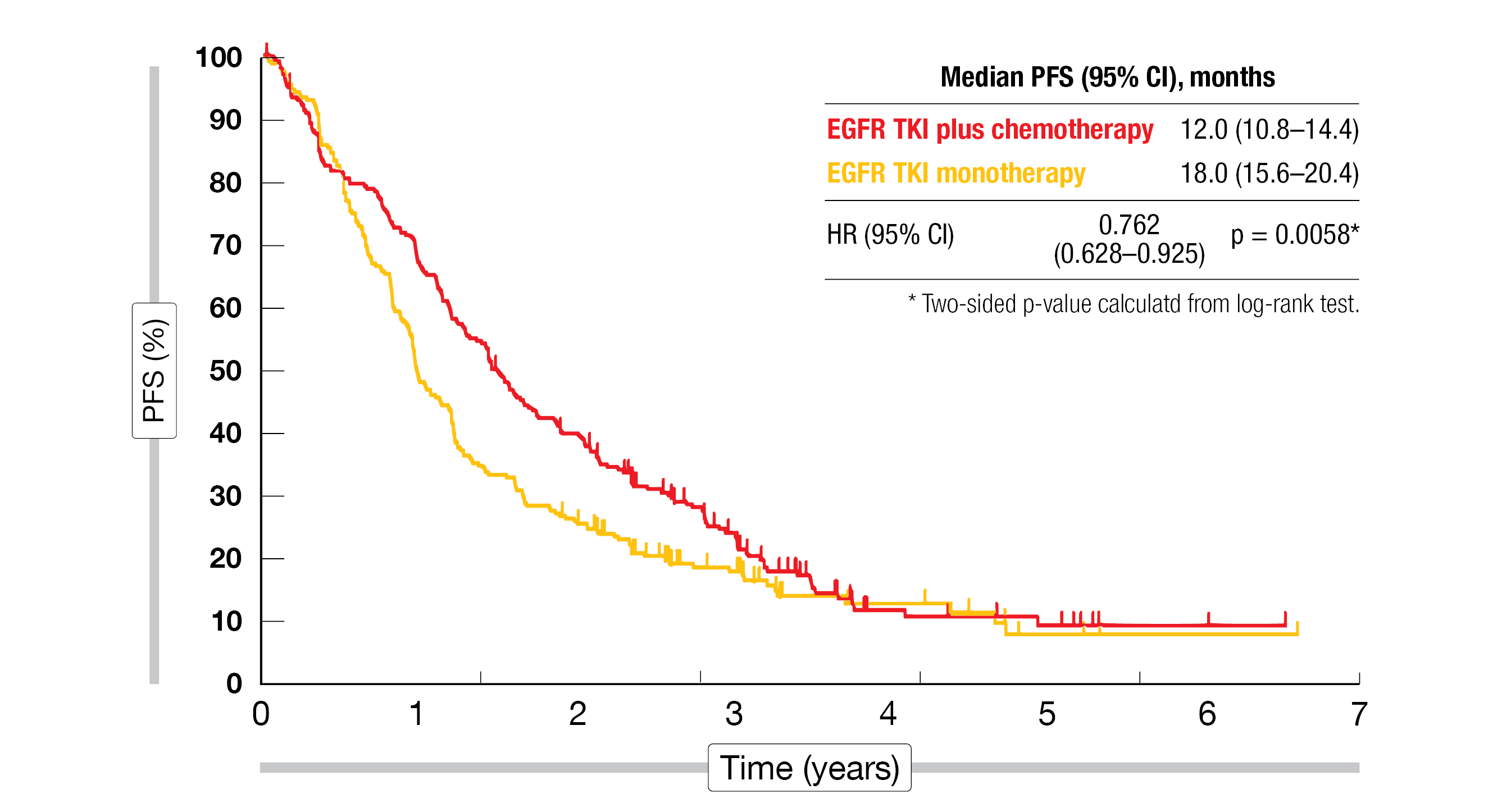
After a median follow-up of 36 months, gefitinib/osimertinib plus inserted chemotherapy significantly improved PFS compared with EGFR TKI monotherapy in the intent-to-treat population (18.0 vs. 12.0 months; HR, 0.762; p = 0.0058; Figure 1). Almost all subgroups favored the experimental approach. The results obtained with gefitinib were compared to those achieved with osimertinib, which showed that both study arms fared considerably better with osimertinib (median PFS, 25.2 vs. 20.4 months; HR, 0.812; p = 0.2475) than with gefitinib (14.4 vs. 9.6 months; HR, 0.687; p = 0.0015).
With respect to the primary endpoint of OS, however, no difference resulted across the two study arms (48.0 months each; HR, 0.985; p = 0.4496). None of the subgroups analyzed appeared to derive significant benefit from the experimental approach. As with PFS, patients treated with osimertinib achieved better OS outcomes in both arms (median OS, not reached in either arm; HR, 0.835; p = 0.5154) than those who received gefitinib (45.6 vs. 43.2 months; HR, 1.016; p = 0.9124). Moreover, no difference regarding the overall response rate (ORR) was observed between the study arms (71.6 % vs. 78.0 %).
Among adverse events (AEs), decreases in neutrophils and platelet counts, nausea and anorexia were of course more frequent with the experimental regimen due to the chemotherapy component. In the experimental arm, AEs leading to treatment discontinuation occurred with gefitinib and osimertinib in 8.4 % and 14.6 %, respectively; in the control arm, this was the case in 14.4 % and 11.3 %, respectively. One patient who received osimertinib monotherapy died due to treatment-related causes. The scientists are currently investigating the mechanisms of acquired resistance using tumor tissue and liquid biopsy samples.
Figure 1: Progression-free survival with EGFR TKI therapy plus inserted chemotherapy vs. EGFR TKI treatment alone
Sunvozertinib: pivotal findings
Approximately 2 % of patients with NSCLC harbor EGFR exon 20 insertion mutations [13]. The EGFR inhibitor sunvozertinib has been developed as an oral, potent, irreversible and selective EGFR inhibitor targeting EGFR exon 20 insertions as well as other EGFR mutations [14]. In the pivotal, single-arm, phase II WU-KONG6 study, patients with locally advanced or metastatic NSCLC and confirmed EGFR exon 20 insertions received sunvozertinib 300 mg OD after 1–3 prior lines of systemic treatment. Their disease had progressed on or after platinum-based chemotherapy. ORR by independent review was defined as the primary endpoint.
The analysis of the WU-KONG6 data presented at ASCO 2023 included 97 patients with 30 different subtypes of EGFR exon 20 insertions after a median of two prior anti-cancer therapies [15]. Sunvozertinib gave rise to an ORR of 60.8 %, which met its predefined target with statistical significance (p < 0.0001). Disease control was achieved in 87.6 %. More than 90 % of patients obtained tumor shrinkage. Responses occurred irrespective of age, sex, smoking status, presence of baseline brain metastases, lines of prior therapy, previous PD-(L)1 treatment, mutation subtypes, and insertion locations. Median duration of response had not been reached yet; 64.4 % of responders were still responding.
Sunvozertinib showed a well-tolerated safety profile that was similar to the safety profiles of other EGFR TKIs. Diarrhea, increased plasma levels of creatine phosphokinase and rash constituted the most common all-grade treatment-emergent AEs. Most of the side effects were grade 1 or 2. Among grade ≥ 3 treatment-emergent AEs, increases in creatine phosphokinase levels ranged first (17.3 %) followed by diarrhea (7.7 %) and anemia (5.8 %). Overall, these data suggest that sunvozertinib is a potential treatment option for patients with NSCLC and EGFR exon 20 insertions. The randomized, multinational phase III WU-KONG28 study is currently evaluating first-line sunvozertinib versus platinum-based chemotherapy in patients with NSCLC and EGFR exon 20 insertions (NCT05668988).
Beamion Lung 1: HER2-positive disease
HER2 mutations are found in lung cancer in 2–4 % of cases, with approximately 50 % classified as HER2 exon 20 insertion mutations that show poor response to TKIs [16-19]. TKIs that target both EGFR and HER are typically limited by toxicities associated with the inhibition of wild-type EGFR [18, 20]. The novel oral TKI BI 1810631 has been designed to target wild-type and mutant HER2 including exon 20 insertions and to avoid toxicity due to the inhibition of wild-type EGFR. It is being tested in tumors with HER2 alterations in the phase I Beamion Lung 1 study. Phase Ia of Beamion Lung 1 is the dose escalation phase and includes patients with solid tumors harboring HER2 aberrations. Heymach et al. presented data for 43 patients including 24 lung cancer patients at ASCO 2023 [21].
Two BI 1810631 doses of 240 mg and 120 mg OD Q3W have been taken into dose optimization. To date, the maximum tolerated dose has not been reached with either the BID or OD schedule. Diarrhea was the most commonly reported AE. The majority of events was graded as 1 or 2, as only four patients developed grade ≥ 3 treatment-related AEs (9.3 %). AEs led to dose reduction and treatment discontinuation in 4.7 % each. Encouraging preliminary activity was observed: NSCLC patients showed objective responses in 46 %, and disease control was achieved in 96 %. In phase Ib of the Beamion Lung 1 study, BI 1810631 will be globally assessed in patients with NSCLC harboring HER2 mutations including exon 20 insertion mutations. Cohort 1 that contains pretreated individuals is ongoing, and four additional NSCLC cohorts are planned.
Predictive biomarkers for amivantamab/lazertinib
The combination of the EGFR-MET bispecific antibody amivantamab and the third-generation EGFR TKI lazertinib has shown activity in cohort A of the CHRYSALIS-2 study that contained patients with EGFR-mutated NSCLC who had progressed after osimertinib and chemotherapy [22]. According to exploratory data, EGFR/MET immunohistochemistry (IHC) staining may predict for responses to this combination. At ASCO 2023, Besse et al. reported results for cohort D of the CHRYSALIS-2 study whose objective was the prospective validation of potential biomarkers based on IHC or ctDNA next-generation sequencing (NGS) [23]. The treatment consisted of amivantamab 1,050 mg (1,400 mg in patients with ≥ 80 kg body weight) i. v. plus lazertinib 240 mg p. o. Prior to study enrollment, the patients had received osimertinib in the first (70 %) or second (30 %) line and were chemotherapy-naïve. Plasma and tissue were collected at baseline. Among 101 response-evaluable patients, 77 had sufficient tissue for MET IHC staining. A predefined Bayesian process allowed for biomarker retraining/validation.
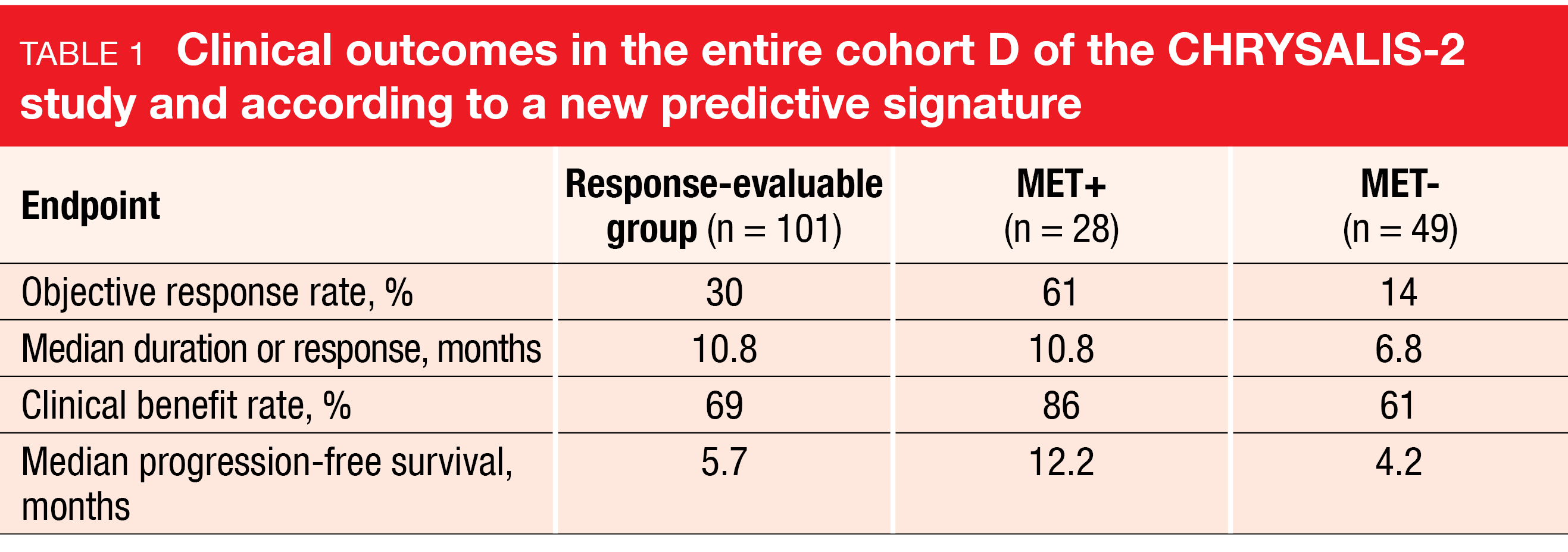
Consistent with previous reports, amivantamab plus lazertinib demonstrated activity with durable responses. In the entire cohort, the ORR was 30 %, and responses lasted for a median of 10.8 months (Table 1). According to the biomarker analysis, a retrained signature of MET 3+ staining on ≥ 25 % of tumor cells (MET+) by IHC was identified as predictive of response to amivantamab and lazertinib regardless of the type of molecular resistance mechanism. In patients with MET+ (n = 28; 36 %), as compared to those with MET- (n = 49; 64 %), the analysis showed advantages regarding ORR, duration of response, clinical benefit rate, and PFS (Table 1). In 17 of the 28 MET+ patients, responses were ongoing at the time of the analysis. Baseline NGS of ctDNA, on the other hand, did not predict responses to amivantamab plus lazertinib.
No new safety signals occurred in the study, and the individual AEs were mostly grade 1 or 2. Treatment-related dose interruptions, reductions, and discontinuations of both drugs were reported in 22 %, 6 %, and 5 %, respectively. In summary, MET+ by IHC might be a predictive biomarker for response to amivantamab plus lazertinib in chemotherapy-naïve patients after osimertinib pretreatment. This marker will be prospectively validated in the CHRYSALIS-2 study.
Primary endpoint of SCARLET: sotorasib plus chemotherapy
The combination of the KRASG12C inhibitor sotorasib with carboplatin/pemetrexed in patients with KRASG12C-mutated NSCLC is being explored in the single-arm, phase II SCARLET study. Thirty patients with advanced, non-squamous NSCLC harboring KRASG12C mutations who are naïve for both KRAS inhibition and cytotoxic chemotherapy are receiving sotorasib 960 mg plus carboplatin AUC 5/pemetrexed 500 mg/m2 Q3W for 4 cycles. The induction phase is followed by sotorasib plus pemetrexed Q3W as maintenance until disease progression.
Sakata et al. reported the primary endpoint of SCARLET, which is ORR by blinded independent central review (BICR), at ASCO 2023 [24]. Indeed, the analysis showed favorable outcomes with an ORR of 88.9 % and ongoing responses in most patients. Changes in target lesions were observed across all PD-L1 expression levels. Median PFS by BICR was 5.7 months; at 6 months, almost 50 % of patients were alive and progression-free. Median OS had not been reached yet, and the 6-month OS rate was 87.3 %. Among treatment-related AEs, anemia as well as decreases in platelet and neutrophil counts were most common, followed by gastrointestinal toxicity. Cytopenias made up the majority of grade ≥ 3 events. One patient died due to grade 5 pneumonia. Dose reductions and interruptions of sotorasib were necessary in 31.0 % and 37.9 %, respectively.
According to a translational analysis based on plasma samples, KRASG12C was still detectable in 50 % of patients at 3 weeks. In this group, responses appeared to be less frequent than in patients who had KRASG12C negativity already at baseline and in those whose KRASG12C allele frequency had decreased during treatment. On the other hand, ORR did not differ according to the presence of TP53, which was the second most common mutation.
Co-alterations in CodeBreaK 200
Sotorasib has demonstrated superior PFS and ORR compared to docetaxel in patients with advanced KRASG12C-mutated NSCLC after ≥ 1 prior treatment line in CodeBreaK 200, which was the first randomized phase III trial of a KRASG12C inhibitor [25]. Given the unmet need to inform treatment decisions using molecular markers, Skoulidis et al. conducted prespecified subgroup analyses of tissue and/or plasma samples from CodeBreaK 200 to identify key genomic alterations [26]. The biomarker-evaluable population comprised 318 patients with tumor and/or plasma NGS data. According to central NGS, co-alterations mainly included TP53 (57 %), STK11 (38 %) and KEAP1 mutations (26 %); these were well balanced across the two treatment arms, which also applied to combinations of STK11 and KEAP1 (17 %). Further aberrations of interest included EGFR mutations (21 %), NTRK fusions (19 %), MET alterations (12 %), ALK translocations (11 %), RET fusions (7 %), ROS1 rearrangements (5 %) and BRAF mutations (5 %).
Sotorasib showed consistent clinical benefit compared to docetaxel regarding both PFS and ORR across the key co-alteration subgroups. PD-L1 expression did not affect the outcomes observed with sotorasib, as the treatment improved PFS over docetaxel in all three PD-L1 categories (i.e., < 1 %, ≥ 1 % and < 50 %, ≥ 50 %). High baseline plasma tumor burden turned out to be a negative prognostic marker irrespective of the type of treatment used in CodeBreaK 200.
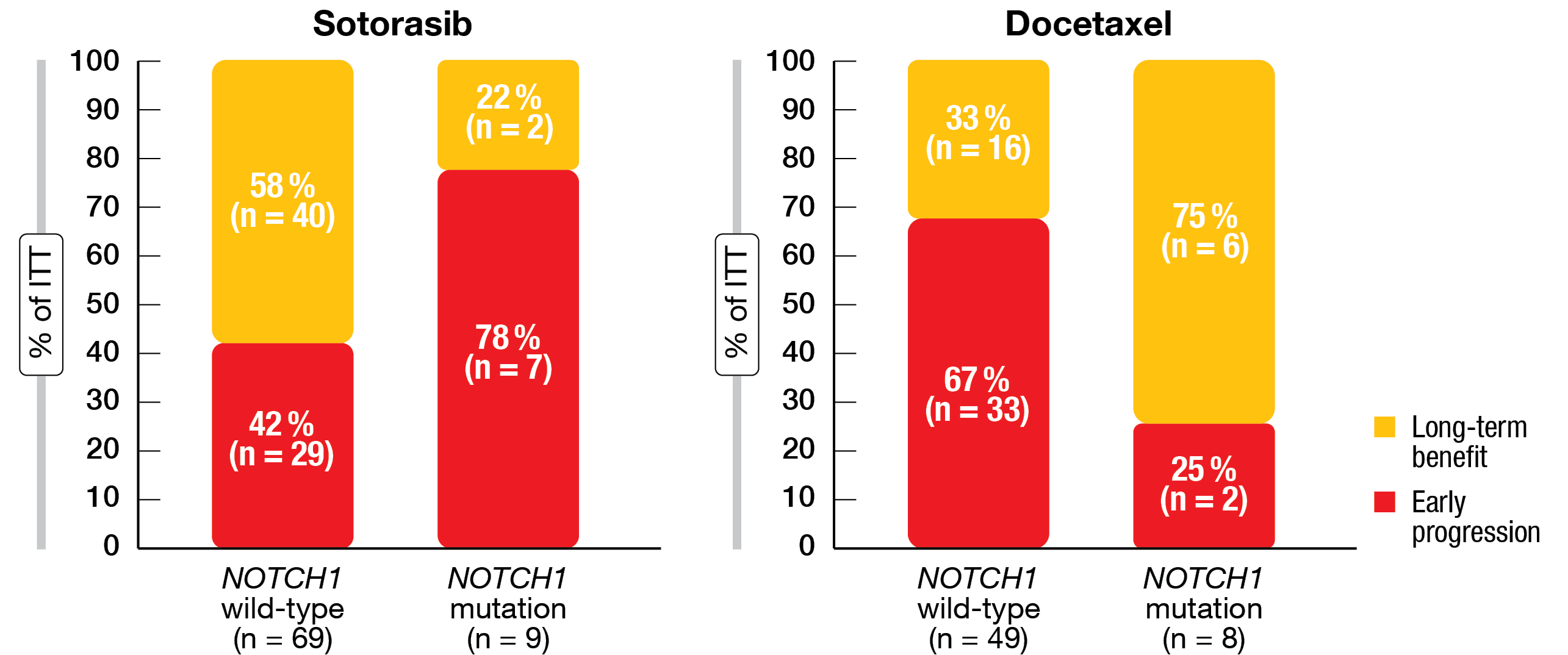
Hypothesis-generating findings included the potential association of non-G12C KRAS co-alterations with primary resistance to either treatment. In this group, neither sotorasib nor docetaxel elicited remissions, and median PFS was 1.8 and 2.5 months with sotorasib and docetaxel, respectively. These results align with preclinical data suggesting that some non-G12C KRAS aberrations mediate sotorasib resistance [27]. Moreover, sotorasib-treated patients with mutated NOTCH1 tended to experience early progression, whereas this was not the case with docetaxel treatment (Figure 2). This signal warrants further exploration. Additional studies will explore and validate the roles of non-G12C KRAS and NOTCH1 co-alterations as potential biomarkers for sotorasib treatment.
Figure 2: Early progression with sotorasib, but not docetaxel, in the presence of NOTCH1 mutations
Encorafenib/binimetinib in BRAFV600E-mutated tumors
The BRAF inhibitor encorafenib in combination with the MEK inhibitor binimetinib has shown clinical efficacy in patients with metastatic BRAFV600E/K-mutant melanoma [28]. In the setting of lung cancer, the ongoing single-arm, open-label, multicenter phase II PHAROS trial is evaluating this regimen in patients with metastatic BRAFV600E-mutant NSCLC after ≤ 1 prior line of treatment. Encorafenib 450 mg OD plus binimetinib 45 mg BID is administered until progression. The study population includes 59 treatment-naïve and 39 previously treated patients. ORR by independent radiologic review represents the primary endpoint.
According to the results reported by Riely et al. at ASCO 2023, the combination demonstrated meaningful clinical benefits with an acceptable safety profile [29]. ORRs were 75 % and 46 % for the treatment-naïve and pretreated cohorts, respectively. Complete remissions resulted in 15 % and 10 %, respectively. In the treatment-naïve group, median duration of response as well as median PFS had not been reached, while in the previously treated group, median duration of response and median PFS were 16.7 months and 9.3 months, respectively. At 24 weeks, 64 % vs. 41 % of patients had obtained disease control.
The safety profile was consistent with that observed in the setting of melanoma. Nausea, diarrhea and fatigue occurred as the most common events. Permanent discontinuation of both encorafenib and binimetinib due to treatment-related AEs resulted in 15 %. The majority of AEs was restricted to grades 1 and 2. Overall, these findings indicate that encorafenib plus binimetinib represents a potential new treatment option for patients with BRAFV600E-mutated metastatic NSCLC.
TRIDENT-1: repotrectinib
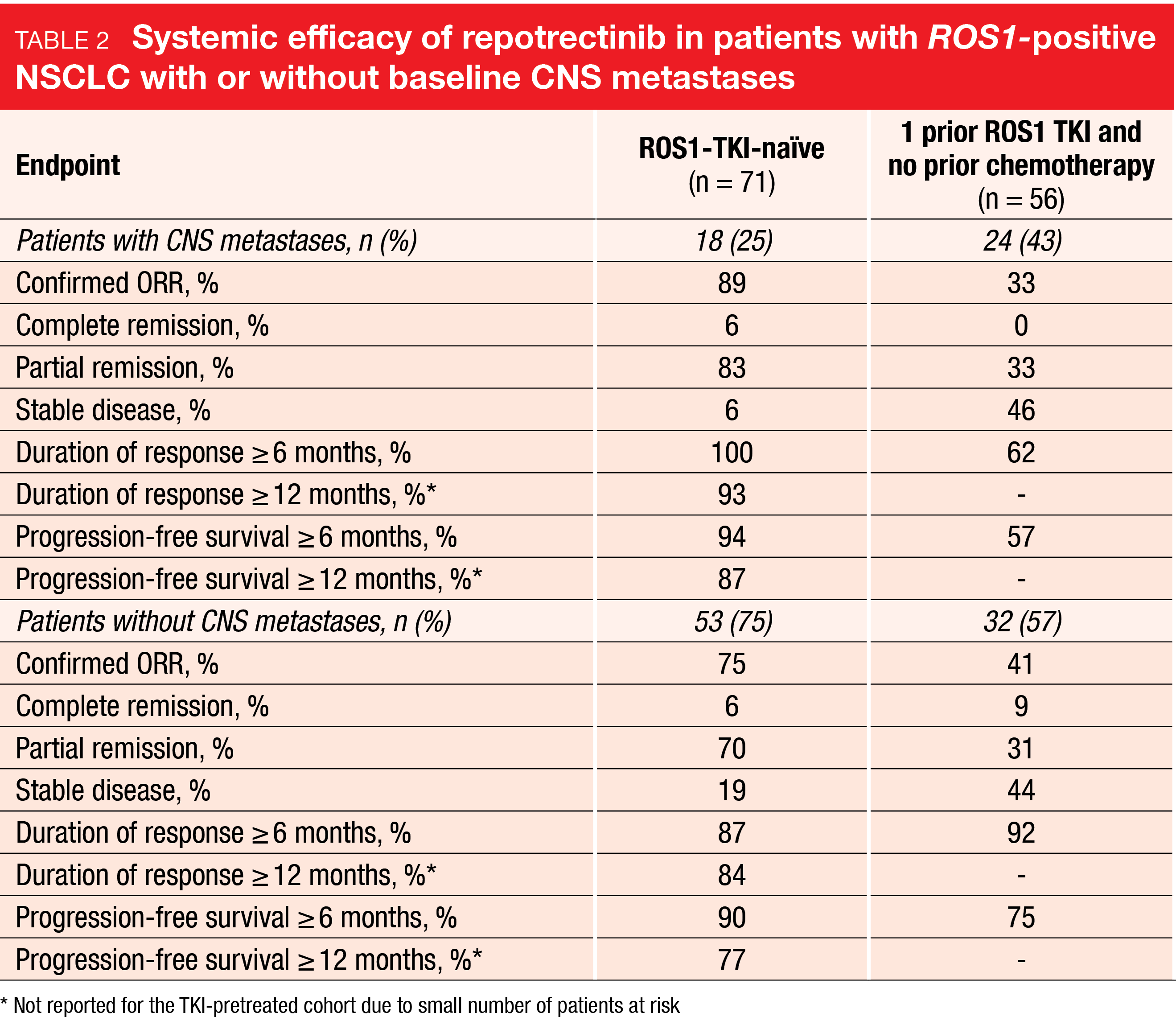
ROS1 TKI therapy is the standard of care in patients with NSCLC harboring ROS1 fusions, although achieving durable benefit remains a challenge with the established agents crizotinib and entrectinib [30-32]. The next-generation TKI repotrectinib, which is selectively active against ROS 1, TRK and ALK, has been developed to improve durability of benefit by decreasing the potential for emergence of resistance mutations and circumventing known resistance mutations [33]. Also, repotrectinib has favorable properties for human brain penetration. This agent is currently under evaluation in the global pivotal phase I/II TRIDENT-1 study that includes four phase II dose expansion cohorts with ROS1-positive advanced NSCLC. Lin et al. reported the first data on repotrectinib in patients with ROS1-positive NSCLC with or without baseline CNS metastases [34]. The efficacy-evaluable population per BICR comprised 71 ROS1-TKI–naïve patients (18 and 53 with and without brain lesions, respectively) and 56 patients who had received one prior ROS1 TKI but no chemotherapy before trial entry (24 and 32 with and without brain lesions, respectively).
Repotrectinib demonstrated durable clinical activity irrespective of ROS1 TKI-pretreatment and CNS status. Confirmed ORRs ranged from 33 % to 89 %, and substantial proportions of patients showed long duration of response as well as freedom from progression (Table 2). In the group with measurable baseline CNS metastases, the treatment induced deep reductions in intracranial tumor burden across the groups. Intracranial responses occurred in 88 % and 42 % in the TKI-naïve and -pretreated cohorts, respectively. Moreover, the CNS activity of repotrectinib proved durable. Intracranial PFS ranged from 0.0+ to 16.4+ months and from 1.6+ to 12.8+ months in the TKI-naïve and -pretreated cohorts, respectively.
The safety profile of repotrectinib was similar in patients with and without CNS metastases, which also applied to nervous system AEs. Side effects leading to treatment discontinuation were reported in 3 % to 10 % across the groups. Dizziness occurred in 57 % and 63 % of patients with and without brain lesions, respectively; these events were mostly grade 1 or 2 and did not necessitate treatment discontinuation. As the authors pointed out, the data from TRIDENT-1 are the first analysis of outcomes on repotrectinib in patients with ROS1-positive NSCLC with or without baseline CNS metastases and suggest that repotrectinib could represent a new treatment option in this population.
REFERENCES
- Hosomi Y et al., Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J Clin Oncol 2020; 38(2): 115-123
- Noronha V et al., Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 2020; 38(2): 124-136
- Giaccone G et al., Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol 2004; 22(5): 777-784
- Herbst RS et al., Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol 2004; 22(5): 785-794
- Piperdi B et al., Schedule-dependent interaction between EGFR-inhibitor (EGFRI) and G2/M blocking agents (G2/MB) on human NSCLC cell lines in vitro. Pros Am Soc Clin Oncol 2004; 23: 7028
- Yamaguchi H et al., Caspase-independent cell death is involved in the negative effect of EGF receptor inhibitors on cisplatin in non-small cell lung cancer cells. Clin Cancer Res 2013; 19(4): 845-854
- Norton L, Simon R, Tumor size, sensitivity to therapy and the design of treatment protocols. Cancer Treat Rep 1976: 61(7): 1307-1317
- Norton L, Simon R, The growth curve of an experimental solid tumor following radiotherapy. J Natl Cancer Inst 1977; 58(6): 1735-1741
- Norton L, Simon R, The Norton-Simon hypothesis revisited. Cancer Treat Rep 1986; 70(1): 163-169
- Kanda S et al., Cytotoxic chemotherapy may overcome the development of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy. Lung Cancer 2015; 89(3): 287-293
- Soria JC et al., Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med 2018; 378(2): 113-125
- Kanda S et al., A phase III study comparing EGFR-TKI monotherapy and EGFR-TKI with inserted cisplatin plus pemetrexed as a first-line treatment in patients with advanced non-squamous non-small cell lung cancer harboring EGFR activation mutation: JCOG1404/WJOG8214L, AGAIN study. J Clin Oncol 41, 2023 (suppl 17; abstr LBA9009)
- Yasuda HY et al., EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012; 13(1): e23-31
- Wang M et al., Sunvozertinib, a selective EGFR inhibitor for previously treated non–small cell lung cancer with EGFR exon 20 insertion mutations. Cancer Discov 2022; 12(7): 1676-1689
- Wang M et al., Sunvozertinib for the treatment of NSCLC with EGFR exon20 insertion mutations: the first pivotal study results. J Clin Oncol 41, 2023 (suppl 16; abstr 9002)
- Baraibar et al., Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit Rev Oncol Hematol 2020; 148: 102906
- Connell CM, Doherty GJ, Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2017; 2(5): e000279
- Robichaux JP et al., Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018; 24(5): 638-646
- Robichaux JP et al., Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 2019; 36(4): 444-457
- Aw DCW et al., Management of epidermal growth factor receptor tyrosine kinase inhibitor-related cutaneous and gastrointestinal toxicities. Asia Pac J Clin Oncol 2018; 14(1): 23-31
- Heymach J et al., Phase I Beamion Lung 1 trial of BI 1810631, a HER2 tyrosine kinase inhibitor, as monotherapy in patients with advanced/metastatic solid tumors with HER2 aberrations: updated data. J Clin Oncol 41, 2023 (suppl 16; abstr 8545)
- Shu CA et al., Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J Clin Oncol 40, 2022 (suppl 16; abstr 9006)
- Besse B et al., Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR-mutated advanced NSCLC in the post-osimertinib setting: Analysis of tissue IHC and ctDNA NGS. J Clin Oncol 41, 2023 (suppl 16; abstr 9013)
- Sakata S et al., A single-arm, phase II study of sotorasib plus carboplatin-pemetrexed in advanced non-squamous, non-small cell lung cancer patients with KRAS G12C mutation (WJOG14821L). J Clin Oncol 41, 2023 (suppl 16; abstr 9006)
- de Langen AJ et al., Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet 2023; 401(10378): 733-746
- Skoulidis F et al., Biomarker subgroup analyses of CodeBreaK 200, a phase 3 trial of sotorasib versus docetaxel in patients with pretreated KRAS G12C-mutated advanced non-small cell lung cancer. J Clin Oncol 41, 2023 (suppl 16; abstr 9008)
- Zhao Y et al., Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 2021; 599(7886): 679-683
- Dummer R et al., Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19(5): 603-615
- Riely GJ et al., Efficacy and safety of encorafenib plus binimetinib in patients with metastatic BRAF V600E-mutant non-small cell lung cancer from the phase 2 PHAROS study. J Clin Oncol 41, 2023 (suppl 16; abstr 9018)
- Dziadziusko R et al., Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small-cell lung cancer. J Clin Oncol 2021; 39(11): 1253-1263
- Shaw T et al., Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer: updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019; 30(7): 1121-1126
- Gainor JF et al., Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol 2017; 2017: PO.17.00063
- Drilon A et al., Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov 2018; 8(10): 1227-1236
- Lin JJ et al., Intracranial and systemic efficacy of repotrectinib in advanced ROS1 fusion-positive non-small cell lung cancer and central nervous system metastases in the phase 1/2 TRIDENT-1 trial. J Clin Oncol 41, 2023 (suppl 16; abstr 9017)
© 2023 Springer-Verlag GmbH, Impressum
More posts
Targeted approaches in advanced disease
Targeted approaches in advanced disease Anti-EGFR agents plus inserted chemotherapy Ac
Small-cell lung cancer: novel agents & biomarkers
Small-cell lung cancer: novel agents & biomarkers Atezolizumab plus talazoparib as
Early-stage NSCLC: current insights into perioperative strategies
Early-stage NSCLC: current insights into perioperative strategies Overall survival sup
Preface – ASCO Lung Cancer 2023
Preface – ASCO Lung Cancer 2023 © private – Manali I. Patel, MD MPH MS, Associate Pro