Stage IV lung cancer: miscellaneous treatment strategies
Portable antimitotic fields
Considering the need for new, well tolerated and effective treatments to improve survival in metastatic NSCLC after platinum-based chemotherapy, Tumor Treating Fields (TTFields) therapy represents an innovative option. TTFields are electric fields that exert physical forces on electrically charged cellular components in dividing cancer cells, thus disrupting cell function [1, 2]. The result is an anti-mitotic effect and induction of immunogenic cell death, which in itself triggers a systemic antitumor immune response [3-5]. This non-invasive locoregional treatment has already been approved in the USA for glioblastoma and malignant pleural mesothelioma [6, 7] and is delivered by a wearable medical device connected to two pairs of adhesive bandages with biocompatible insulated ceramic discs that are placed on the chest. A pilot study has demonstrated the safety and feasibility of this approach in advanced NSCLC [9]. The patients are able to perform their regular activities of daily living during TTFields therapy, while all other treatments and patient care continue to be provided.
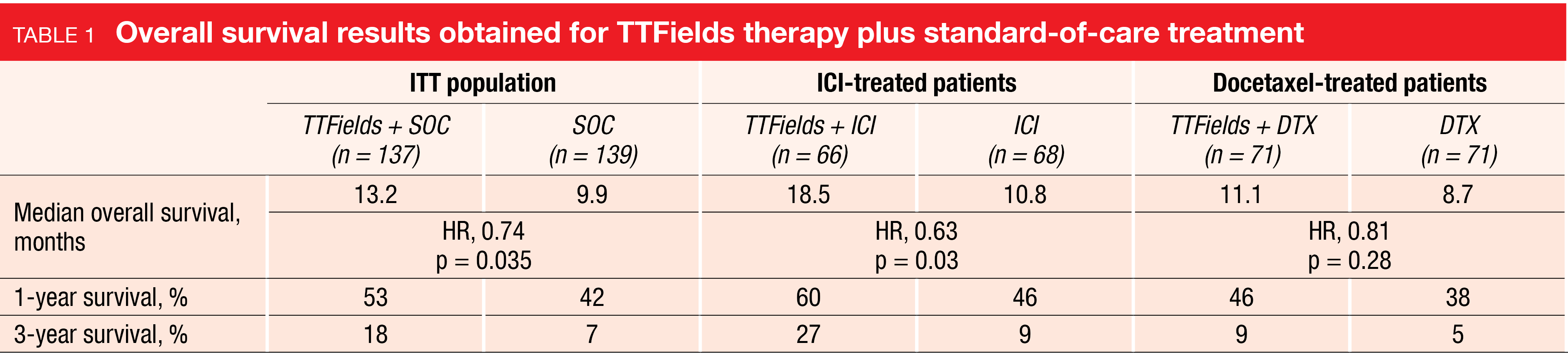
Preclinical NSCLC models have suggested that TTFields therapy amplifies effects of immune checkpoint inhibitors (ICIs) and taxanes [4, 5, 8]. The randomized phase III LUNAR study was initiated to evaluate the safety and efficacy of TTFields therapy together with standard-of-care (SOC) treatment vs. SOC alone in patients with metastatic NSCLC progressing on or after platinum-based therapy. Overall, 137 and 139 patients were analyzed in the experimental and control arms; within either group, approximately half had received ICI as SOC treatment, and the other half had been treated with docetaxel. Leal et al. reported the findings at the ASCO 2023 Congress [10].
More favorable findings in the ICI group
Median exposure to treatment was longer in the experimental arm than in the control arm (14.6 vs. 10.3 weeks), with mean TTFields therapy duration being approximately 2-fold greater in the ICI group than in the docetaxel group. Regarding the primary endpoint of overall survival (OS), the TTFields treatment in addition to SOC induced a statistically and clinically significant 3-month improvement compared to SOC alone in the ITT population (median OS, 13.2 vs. 9.9 months; HR, 0.74; p = 0.035; Table 1). In the subgroup of ICI-treated patients, this difference was almost as large as 8 months (18.5 vs. 10.8 months; HR, 0.63; p = 0.03), thus exceeding the survival benefit observed for docetaxel-treated individuals who showed a trend favoring the combined approach (Table 1). Progression-free survival (PFS) did not differ across the two arms in the ITT population (4.8 vs. 4.1 months; HR, 0.85; p = 0.23), which also applied to the overall response rates (ORR; 20 % vs. 17 %; p = 0.5). Five complete responses occurred, which included four with TTFields and one with SOC alone; all of these were observed in ICI-treated patients.
TTFields therapy did not add any systemic toxicity to SOC therapy. Dermatitis was the most frequent treatment-emergent adverse event (AE) in the experimental arm (all grades, 43 % vs. 2 %), followed by fatigue (28 % vs. 37 %) and musculoskeletal pain (36 % vs. 27 %). In the majority of cases, dermatitis was restricted to grade 1 or 2. No grade 4 or 5 toxicities were attributable to TTFields therapy. The authors stated in their summary that TTFields therapy should be considered part of SOC for advanced metastatic NSCLC. Additional pivotal studies evaluating TTFields therapy plus SOC as first-line treatment of metastatic NSCLC as well as early-stage disease are planned.
Dato-DXd + pembrolizumab ± chemotherapy
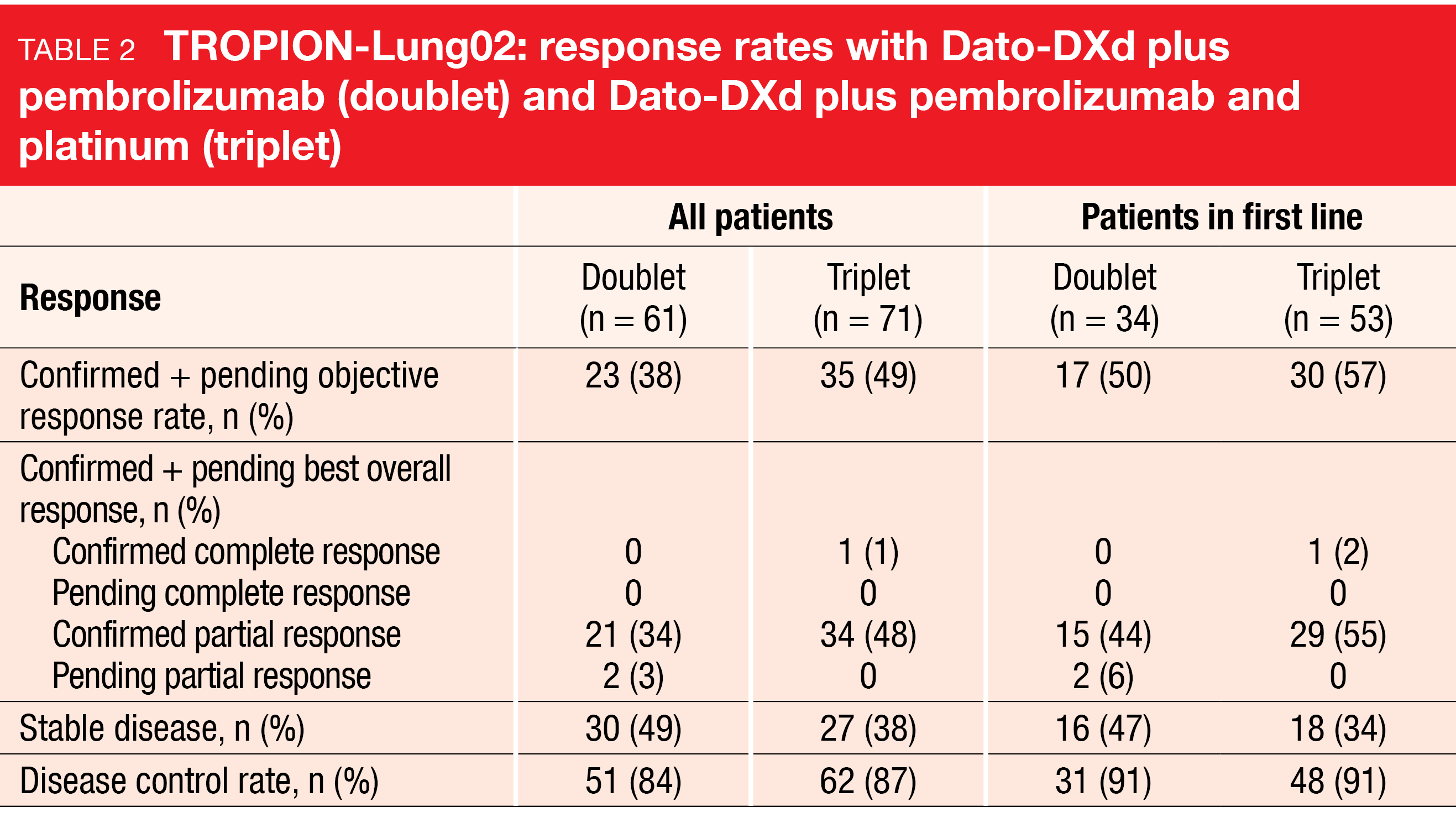
Datopotamab deruxtecan (Dato-DXd), an antibody-drug conjugate containing a TROP2-directed antibody linked to a highly potent cytotoxic payload, has demonstrated encouraging activity as monotherapy in patients with heavily pretreated advanced NSCLC [11]. Based on the assumption that the efficacy of Dato-DXd might be increased by the addition of combination partners, the phase Ib TROPION-Lung02 study was conducted to assess doublet therapy with Dato-DXd plus the PD-1 antibody pembrolizumab (cohorts 1 and 2) and triplet therapy with Dato-DXd plus pembrolizumab and carboplatin or cisplatin (cohorts 3-6) at different doses. TROPION-Lung 2 is the first study to evaluate these regimens in advanced NSCLC without actionable genomic alterations. Sixty-four and 72 patients with advanced/metastatic NSCLC received the doublet and triplet regimens, respectively, with 37 and 54, respectively, being treated in the first-line setting. Immunotherapy had previously been administered in the entire group in 19 % and 25 %, respectively.
In both the first line and later lines, Dato-DXd plus pembrolizumab with or without chemotherapy showed encouraging efficacy (Table 2) [12]. Confirmed plus pending ORR was 38 % and 49 % for the doublet and triplet groups, respectively; in the first line, ORRs of 50 % and 57 %, respectively, were achieved. Disease control rates were 84 % and 87 %, respectively, in all patients. Median duration of response had not been reached in either group, and the treatment gave rise to deep responses. Many patients obtained tumor burden reduction regardless of their PD-L1 expression status. The preliminary median PFS in all patients was 8.3 and 7.8 months, respectively.
During the dose-finding phase, two patients receiving Dato-DXd plus pembrolizumab and chemotherapy experienced dose-limiting toxicities, which were neutropenia and thrombocytopenia. Finally, Dato-DXd 6 mg/kg proved to be safe in each combination. Treatment-related AEs grade ≥ 3 emerged in 31 % and 58 % with the doublet and triplet regimens, respectively. In 23 % and 28 %, respectively, Dato-DXd was discontinued due to AEs. No treatment-related deaths occurred. The most frequent AEs of any grade comprised stomatitis, nausea, anemia, and fatigue. Hematologic AEs, particularly those of grade ≥ 3, were more frequently observed with triplet than with doublet therapy.
Among AEs of special interest, oral mucositis/stomatitis was the most common event but was predominantly grade 1 or 2. Interstitial lung disease(ILD)/pneumonitis occurred in 17 % and 22 % in the doublet and triplet groups, respectively, with 3 % each graded as ≥ 3. No grade 4 or 5 adjudicated ILD/pneumonitis events were observed. At present, Dato-DXd plus pembrolizumab with or without chemotherapy is being compared with first-line SOC regimens in the pivotal phase III TROPION-Lung07 (NCT5555732) and TROPION-Lung08 (NCT05215340) trials.
KEYNOTE-789: IO after failure of EGFR TKIs
ICI therapy has defined new standards in the setting of metastatic NSCLC without driver alterations. However, pembrolizumab and other ICIs have also shown activity in TKI-resistant, EGFR-mutant NSCLC [13-15]. To extend the benefit of ICIs in this population, pembrolizumab plus chemotherapy was evaluated in patients with stage IV non-squamous NSCLC with EGFR deletion 19 or L858R mutation and disease progression after TKI therapy in the randomized phase III KEYNOTE-789 trial. The patients had progressed either after first-/second-generation EGFR TKI treatment without T790M resistance mutation or with T790M mutation and osimertinib failure, or had experienced osimertinib failure in the first-line setting regardless of T790M status. Patients in the experimental arm (n = 245) received pembrolizumab 200 mg plus pemetrexed and carboplatin or cisplatin Q3W for 4 cycles followed by pembrolizumab 200 mg Q3W for 31 cycles plus pemetrexed Q3W. In the control arm (n = 247), placebo was administered in addition to the same chemotherapy regimen. The design contained an optional crossover from the control arm to pembrolizumab 200 mg Q3W for 35 cycles in case of progression. PFS by blinded independent central review and OS were defined as the dual primary endpoint.
According to the data presented by Yang et al. at ASCO 2023, the addition of pembrolizumab to chemotherapy prolonged PFS and OS, although the results did not reach statistical significance per the prespecified statistical analysis plan [16]. PFS findings at the time of the second interim analysis showed a 20 % risk reduction (median PFS, 5.6 vs. 5.5 months; HR, 0.80; p = 0.0122) with 12-month rates of 14.0 % vs. 10.2 % and 24-month rates of 4.7 % vs. 3.5 %. Median OS at the time of the final analysis was 15.9 vs. 14.7 months (HR, 0.84; p = 0.0362). No subgroup derived a particularly pronounced OS benefit from the ICI-based therapy. However, patients with PD-L1–positive tumors (TPS ≥ 1 %) experienced greater mortality reduction (median OS, 18.6 vs. 14.1 months; HR, 0.77) than those without PD-L1 expression (TPS < 1 %; 15.7 vs. 14.7 months; HR, 0.91).
ORRs were 29.0 % vs. 27.1 % for pembrolizumab plus chemotherapy vs. chemotherapy alone, and responses lasted for a median of 6.3 vs. 5.6 months. At 9 months, ongoing responses were found in 34.0 % vs. 22.9 %. The AEs presented themselves manageable, with no new safety signals identified. Treatment-related grade 3–5 AEs occurred in 43.7 % vs. 38.6 %. Discontinuation of pembrolizumab or placebo resulted in 9.8 % vs. 4.5 %. Taken together, the findings from the KEYNOTE-789 study are consistent with prior data showing that the benefit obtained with anti-PD-(L)1–based treatment is smaller in patients with TKI-resistant, EGFR-mutant metastatic NSCLC than in those with EGFR wild-type tumors. As the authors noted, additional biomarker research is required to determine which of these patients might benefit as there remains a great unmet need for this population.
Cohort C of VARGADO by KRAS and PD-L1 status
Cohort C of the ongoing, prospective, non-interventional VARGADO study is investigating anti-angiogenic treatment with nintedanib in addition to docetaxel after first-line ICI plus chemotherapy in patients with adenocarcinoma of the lung. At ASCO 2023, Grohé et al. reported an updated analysis of Cohort C (n = 219) that focused on the safety and efficacy of the nintedanib combination according to PD-L1 and KRAS mutational status [17].
In the overall population, median PFS and OS were 4.5 and 9.6 months, respectively. Among patients with documented KRAS mutation status (n = 119), 75 had KRAS wildtype. In this group, median PFS and OS were 3.7 and 7.7 months, respectively. For the cohort with KRAS mutations, median PFS and OS were 5.1 and 6.7 months, respectively. Non-G12C KRAS mutations were found in 33 patients; here, median PFS and OS were 5.0 and 6.7 months, respectively. Likewise, ORR did not differ greatly across all of these groups (range, 18.2 % to 27.3 %). The corresponding disease control rates ranged from 45.3 % to 48.5 %. Also, the PD-L1 expression status prior to the first-line treatment did not affect OS (10.6 and 10.5 months for PD-L1 expression < 1 % and ≥ 1 %, respectively) or disease control rates (51.2 % and 51.3 %, respectively). AEs related to nintedanib mostly included diarrhea (any grade, 34.7 %), nausea (14.2 %), vomiting (8.2 %) and fatigue (7.8 %). The authors concluded that in patients with adenocarcinoma of the lung, nintedanib plus docetaxel is an effective and safe second-line treatment option after ICI plus chemotherapy independent of PD-L1 expression and KRAS mutation status.
REFERENCES
- Mun EJ et al., Tumor-Treating Fields: A fourth modality in cancer treatment. Clin Cancer Res 2018; 24(2): 266-275
- Giladi M et al., Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep 2015; 5: 18046
- Voloshin T et al., Tumor Treating Fields (TTFields) hinder cancer cell motility through regulation of microtubule and actin dynamics. Cancers (Basel) 2020; 12(10): 3016
- Voloshin T et al., Tumor-Treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother 2020; 69(7): 1191-1204
- Barsheshet Y et al., Tumor Treating Fields (TTFields) concomitant with immune checkpoint inhibitors are therapeutically effective in non-small cell lung cancer (NSCLC) in vivo model. Int J Mol Sci 2022; 23(22): 14073
- Stupp R et al., NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012; 48(14): 2192-2020
- Stupp R et al., Effect of Tumor-Treating Fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017; 318(23): 2306-2316
- Giladi M et al., Alternating electric fields (Tumor-Treating Fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin Oncol 2014; 41 Suppl 6: S35-S41
- Pless M et al., A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 2013; 81(3): 445-450
- Leal T et al., Tumor Treating Fields (TTFields) therapy with standard of care in metastatic non-small cell lung cancer following platinum failure: randomized, phase 3 LUNAR study. J Clin Oncol 41, 2023 (suppl 17; abstr LBA9005)
- Garon EB et al., TROPION-PanTumor01:
updated results from the NSCLC cohort of the phase 1 study of datopotamab deruxtecan in solid tumors. WCLC 2021, MA03.02 - Goto Y et al., TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab with or without platinum chemotherapy in advanced non-small cell lung cancer. J Clin Oncol 41, 2023 (suppl 16; abstr 9004)
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-1550
- Reck M et al., Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019; 7(5): 387-401
- Gettinger S et al., Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol 2018; 13(9): 1363-1372
- Yang J CH et al., Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor-resistant, EGFR-mutant, metastatic non-squamous NSCLC: phase 3 KEYNOTE-789 study. J Clin Oncol 41, 2023 (suppl 17; abstr LBA9000)
- Grohé C et al., Impact of PD-L1 and KRAS status on the efficacy of nintedanib + docetaxel following treatment with first-line immune checkpoint inhibitor + chemotherapy in patients with adenocarcinoma NSCLC: Analysis of cohort C of the non-interventional VARGADO trial. J Clin Oncol 41, 2023 (suppl 16; abstr e21149)
© 2023 Springer-Verlag GmbH, Impressum
More posts
Targeted approaches in advanced disease
Targeted approaches in advanced disease Anti-EGFR agents plus inserted chemotherapy Ac
Small-cell lung cancer: novel agents & biomarkers
Small-cell lung cancer: novel agents & biomarkers Atezolizumab plus talazoparib as
Early-stage NSCLC: current insights into perioperative strategies
Early-stage NSCLC: current insights into perioperative strategies Overall survival sup
Preface – ASCO Lung Cancer 2023
Preface – ASCO Lung Cancer 2023 © private – Manali I. Patel, MD MPH MS, Associate Pro