New clinical insights in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) affected approximately 905,000 new diagnosed cases worldwide in 2020 and showed a high mortality rate [1]. Current first-line treatment for advanced HCC includes atezolizumab plus bevacizumab [2], as well as the tyrosine kinase inhibitors sorafenib [3, 4] and lenvatinib [5].
Pembrolizumab monotherapy: updated analysis of the KEYNOTE-224 trial
Previously reported data of the KEYNOTE-224 single-arm, non-randomized, multicenter, open-label, phase II study (NCT02702414) in advanced HCC have shown that pembrolizumab monotherapy has a durable antitumor activity and a manageable safety profile in sorafenib-pretreated (cohort 1) and treatment-naive (cohort 2) patients. At this year’s ASCO meeting, the 3-year follow-up data of cohort 2 were presented [6].
Previously untreated HCC patients enrolled in cohort 2 of the KEYNOTE-224 study presented with the following eligibility criteria: histologically, cytologically, or radiologically confirmed advanced HCC; Barcelona Clinic Liver Cancer (BCLC) stage C or B not amenable or refractory to locoregional therapy, and not amenable to curative treatment; Child-Pugh liver function class A; measurable disease per RECIST v1.1 by blinded independent central review (BICR); and ECOG PS 0 or 1. Pembrolizumab (200 mg) was given intravenously (IV) every three weeks (Q3W) for ≤35 cycles (approximately 2 years). The primary endpoint of the study was the objective response rate (ORR), while the duration of response (DoR), the disease control rate (DCR), the time to progression (TTP), the progression-free survival (PFS), the overall survival (OS), the safety and the tolerability were assessed secondarily.
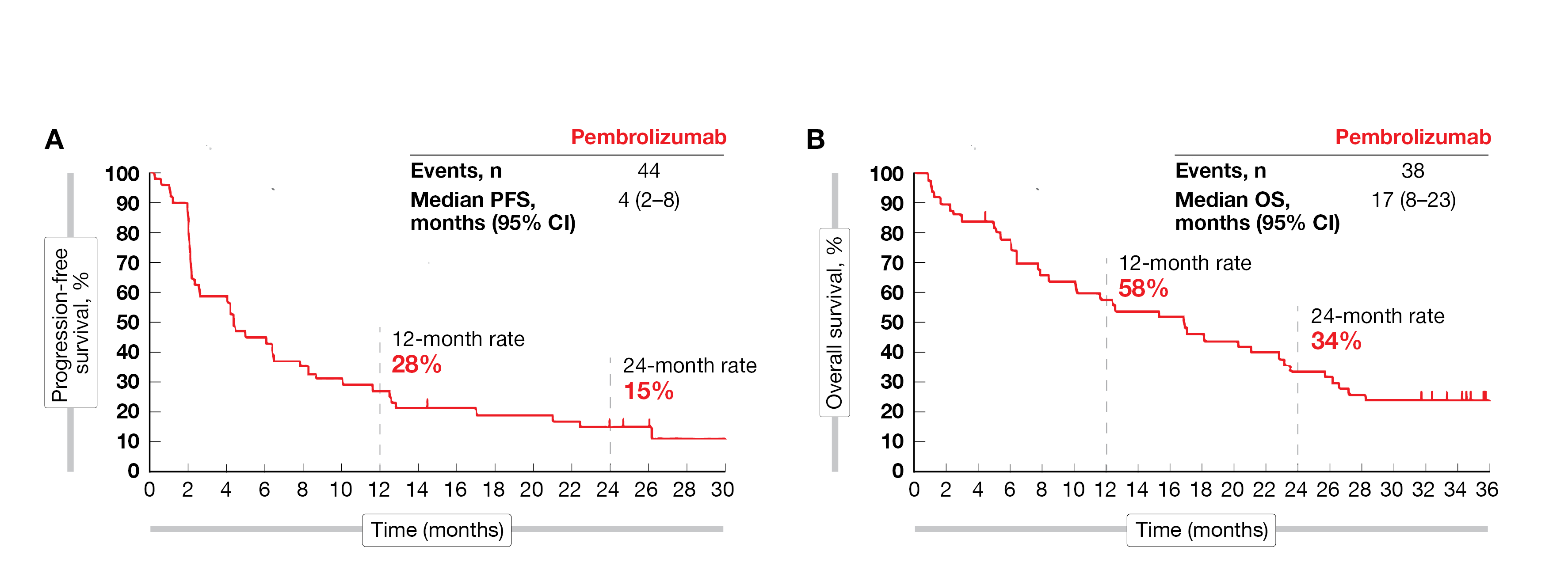
The median follow-up of this new analysis – defined as the time from first dose to data cut-off (October 1, 2021) – was 35 months. All 51 patients recruited in this study received at least one dose of pembrolizumab. The ORR was 16 % (95 % CI, 7 - 29), including eight patients with a partial response (PR); to note, the ORR was independent of a viral or non-viral etiology of HCC. The DCR reached 57 % (95 % CI, 42 - 71). The median DOR was not reached (NR; range, 3 - 24+) at the time of this analysis but 58 % of responders were estimated to have a response duration of more than 18 months. The median time to progression was four months (95 % CI, 3 - 9), the median PFS was four months (95 % CI, 2 - 8) and the estimated 24-month PFS was 15 % (Figure 1). The median OS reached 17 months (95 % CI, 8 - 23) and the estimated OS rate at 24-month was 34 %.
In total, 55 % of patients experienced treatment-related adverse events (TRAEs) of any grade and 16 % of grade 3 to 5. The most common grade ≥ 3 TRAEs observed were myalgia and abdominal pain (2 % each).
According to the updated results from cohort 2 of the KEYNOTE-224 study, pembrolizumab monotherapy continued to demonstrate a durable antitumor activity, a promising OS, and a manageable safety profile in patients with advanced HCC without prior systemic therapy.
Figure 1: Progression-free survival (A) and overall survival (B) in the KEYNOTE-224 study (updated analysis).
Combined therapy in advanced unresectable or metastatic HCC
In an open-label, single-arm, multicenter, phase II study (NCT04542837) combining KN046 – a bispecific antibody targeting both anti-PD-1/PD-L1 and CTLA-4/B7 immune checkpoint pathways – and lenvatinib – a small-molecule tyrosine kinase inhibitor – showed good efficacy and a tolerability as treatment of advanced unresectable or metastatic HCC [7]. The data set has recently been updated, as more patients enrolled in the study with a longer follow-up duration [8].
In this phase II trial recruited patients with unresectable or metastatic HCC wo had a BCLC stage B or C not suitable for curative surgery or local therapy, received lenvatinib orally (12 mg/day for a body weight [BW] ≥ 60 kg or 8 mg/day for a BW < 60 kg) and KN046 (5 mg, IV, on Day 1 of a 21-day cycle) until disease progression, intolerable toxicity or 2-year treatment.
The co-primary endpoints – safety and ORR by RECIST v1.1 per investigators – were met. In total, 55 enrolled patients received a combination of KN046 and lenvatinib with a median duration of 25 weeks; the ORR reached 51.9 % (95 % CI, 37.6 – 66.0) and the DCR was 86.5 % (95 % CI, 74.2-94.4). The median PFS was 9.3 months (95 % CI, 7.0 – not estimable [NE]).
TRAEs were recorded in 98.2 % of patients with decreased platelet count (7.3 % of patients) and increased aspartate aminotransferase (3.6 %) being the most frequent grade ≥ 3 TRAEs.
The novel combined therapy, KN046 plus lenvatinib, demonstrated a clinical benefit in ORR and PFS, as well as a manageable safety profile as first-line treatment of advanced unresectable or metastatic HCC.
RATIONALE-208: Novel option in advanced HCC…
Patients with advanced HCC present an unmet medical need beyond the first-line treatment. Tislelizumab (TIS) is a novel anti-PD-1 antibody that has been successfully investigated as monotherapy or in combination with chemotherapy in various malignancies, like locally advanced or metastatic esophageal squamous cell carcinoma [9], non-small cell lung cancer [10] or previously treated advanced HCC [11]. TIS has been engineered to limit antibody-dependent cellular phagocytosis (ADCP), a potential mechanism of resistance to anti-PD-1 therapies [12, 13]. As first-line treatment for advanced HCC, sorafenib (SOR) or lenvatinib (LEN) continue to be an important part of the clinical armamentarium, even considering recent approval of new immune-oncology-based combinations (e.g., atezolizumab and bevacizumab). In the open-label, multicenter, phase II RATIONALE-208 study (NCT03419897), tislelizumab was clinically active and generally well tolerated in patients with previously treated advanced HCC [14]. A descriptive-only secondary analysis of patients with advanced HCC who had been previously treated with at least one prior line of systemic therapy (SOR/LEN) and had received at least one dose of TIS (200 mg, IV, Q3W) was presented at ASCO 2022 meeting [14]. Clinical activity was evaluated by an independent review committee (IRC) through ORR, DoR, PFS and OS.
After a median follow-up of 12.5 months for patients previously treated with SOR/LEN, IRC-confirmed ORR was 13.6 % (95 % CI, 9.5 – 18.7), including two patients (0.9 %) with a complete response (CR) and 30 patients (12.8 %) with a PR. Overall, 55.3 % of the patients (95 % CI, 48.7 – 61.8) achieved disease control, while the median DoR was not reached at the time of the analysis. The median PFS by IRC was 2.7 months (95 % CI, 1.6 – 2.8) and the median OS was 13.5 months (95 % CI, 10.9 – 15.8) in all treated patients.
Safety data in this study indicated that tislelizumab was generally well tolerated in patients previously treated with SOR/LEN. Overall, 49.4 % of patients had grade ≥ 3 TEAEs, the most common being increased aspartate aminotransferase (AST) (26.0 %), alanine aminotransferase (ALT) (19.6 %), increased blood bilirubin (18.3 %), decreased appetite (16.6 %) and asthenia (16.6 %). Immune-related AEs were experienced by twelve patients (5.1 %), the most frequently reported being hypothyroidism (6.8 %) and hyperthyroidism (2.6 %). Hepatic-related immune-mediated TEAEs reported in ≥ 1 % of patients included increased AST in 4 patients, and increased ALT as well as hepatitis in 3 patients each.
Tislelizumab was clinically active and well tolerated in patients with advanced HCC who have received prior systemic treatment with SOR/LEN. TIS represents an effective second- or third-line therapeutic option.
…and evaluation of hepatitis B virus DNA during tislelizumab treatment
Whether or not TIS treatment is associated with an increase in hepatitis B virus (HBV) DNA, as well as the clinical significance of HBV DNA elevations, during or after tislelizumab treatment is currently being explored in the ongoing phase II RATIONALE-208 trial (NCT03419897) [14].
So far, all 249 patients enrolled in this study had at least one prior systemic therapy for advanced HCC; all patients received TIS (200 mg, IV, Q3W). Approximately half of the recruited patients (n = 128) had a history of HBV infection. Patients with inactive, chronic, or active HBV were eligible if HBV DNA levels were less than 500 IU/mL at screening (patients with detectable hepatitis B surface antigen [HBsAg] or detectable HBV DNA were required to be managed per treatment guidelines). HBV DNA testing was conducted every four cycles if HBV DNA was detectable at screening, or when clinically indicated. The ORR assessed by an IRC in patients with a history of HBV infection was consistent with the ORR observed in the overall population (12.5 % vs 13.3 %, respectively). First results demonstrated that among the 114 patients who were HBsAg positive at baseline (BL), 36 had detectable HBV DNA at BL, and 32 had detectable HBV DNA and HBsAg at BL. In seven patients, clinically significant increased HBV DNA levels were observed compared to BL, independently of the time of TIS initiation. All seven patients were HBsAg positive at BL, had been receiving antiviral treatment for at least 3 months before the first dose of TIS, six of them showed increased ALT levels compared to BL during the study and four of them had at least a 3-fold increase in ALT, observed concurrently or soon after HBV DNA increases.
IRC-assessed best overall response (BOR) was a PR for one patient, as well as increased HBV DNA and progressive disease for the remaining six patients. HBV-related TEAEs were reported in six of the seven treated patients (grade 3 TEAE of hepatitis B, n = 2; grade 2 TEAE of HBV reactivation, n = 2; increased HBV DNA, with one grade 1 and one grade 3 event, n = 2). All HBV-related TEAEs were non-serious and did not result in discontinuation of TIS.
In this preliminary trial, the clinically significant increases in HBV DNA from BL were reported in a small number of patients; therefore, this does not suggest that TIS is associated with increased HBV DNA. Additionally, HBV DNA increases did not impact the treatment, as tumor responses in these patients were consistent with the overall population and HBV-related TEAEs were manageable. The effect of TIS in patients with HBV infection will be further evaluated in a currently ongoing phase III trial (NCT03412773).
AdvanTIG-206: Dual targeting with anti-TIGIT and anti-PD-1 antibodies
Atezolizumab plus bevacizumab represents the new standard of care in systemic front-line treatment of patients with advanced HCC [16]. However, novel options are needed to further improve overall survival and quality of life in this patient population. In preclinical studies, dual combination with anti-TIGIT and anti-PD-1 has shown synergistic inhibition of tumor growth [17]. Moreover, BAT1706,a biosimilar of bevacizumab – an anti-VEGF antibody – has been described to improve OS rate in HCC [18].
AdvanTIG-206 is a randomized, multicenter, open-label, phase II (NCT04948697) study set out to explore triple targeting of tumors with an anti-TIGIT (ociperlimab, 900 mg, IV, Q3W), an anti-PD-1 (tislelizumab, 200 mg, IV, Q3W) and an anti-VEGF (BAT1706, 15 mg/kg, IV, Q3W) [19]. Eligible patients will be randomized 2:1 either in Arm A to receive the triple therapy or in Arm B for the dual combination of tislelizumab and BAT1706. Eligible patients must have a histologically confirmed HCC (BLCL stage C or stage B that is not amenable to curative treatment), at least one measurable lesion, an ECOG PS of 0 or 1 and no prior systemic therapy. All patients will be treated until loss of clinical benefit or unacceptable toxicity assessed by the investigator.
The primary endpoint is the ORR as evaluated by the investigator according to RECIST v1.1. The DoR, the TTR, the DCR, the clinical benefit rate (CBR), the PFS, the OS, as well as the safety and the tolerability are the secondary endpoints. The aim of this study is to enroll approximately 90 patients with unresectable HCC.
Evaluation of low dose apatinib
Recently, apatinib – a tyrosine kinase inhibitor – has shown promising antitumoral activity in the management of second- or later line of therapy in patients with advanced HCC [20]. At this year’s ASCO meeting, a study analyzing the efficacy and safety of low dose apatinib in patients with advanced HCC was presented [21].
Among the 178 patients with advanced HCC enrolled in this real-world study (Chi82170369), 174 received a low dose of oral apatinib (250 mg daily) until disease progression, while four patients were administered a higher dose (500 mg daily). Moreover, 25 patients were also treated with immunotherapy and 103 patients received additional transarterial chemoembolization (TACE) at least once. The endpoints analyzed were tumor response, PFS, OS and safety.
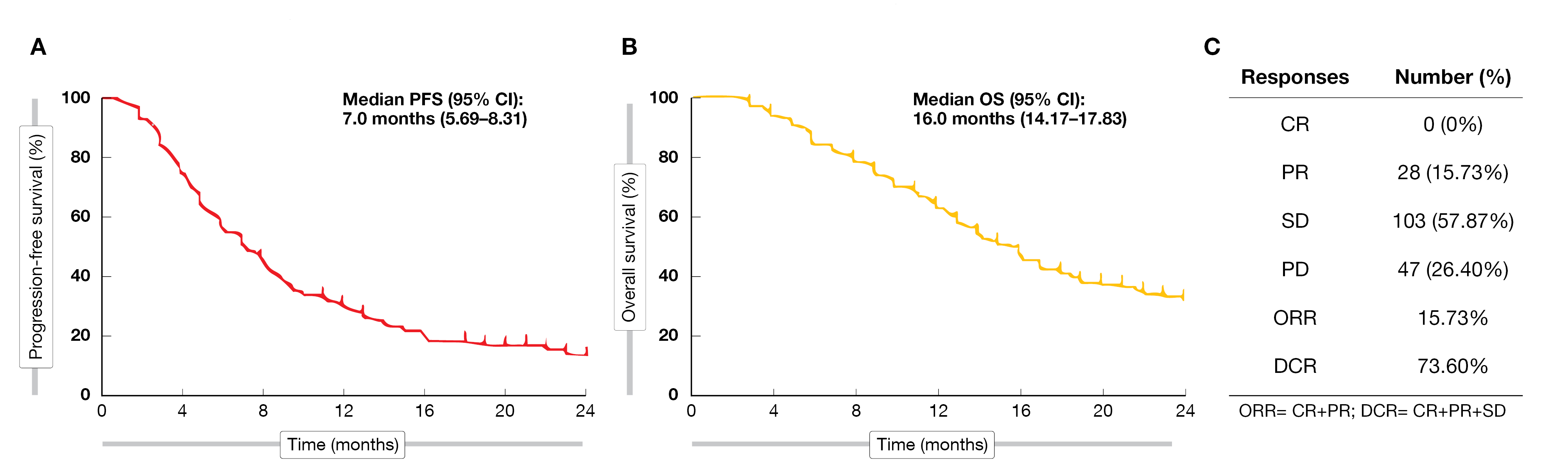
During the 24-month follow-up period, the ORR reached 15.7 % (all patients showing a PR) and the DCR was 73.6 %. (Figure 2). Among the 28 patients with a PR, 27 received apatinib as first- or second-line therapy. Moreover, 21 had a combined treatment with immunotherapy or TACE, indicating early application of apatinib and combination treatment could provide better efficacy. The median OS was 16.0 months and the median PFS was 7.0 months, respectively. A multivariable analysis confirmed that third-line therapy (HR = 3.21; 95 % CI, 1.54 – 6.68; p = 0.002) and portal vein tumor thrombus (HR = 1.75; 95 % CI, 1.13 – 2.70; p = 0.011) were significantly associated with a worse PFS. On the contrary, apatinib combined with immunotherapy (HR = 0.52; 95 % CI, 0.32 – 0.83; p= 0.008) or TACE (HR = 0.27; 95 % CI, 0.18 – 0.40; p< 0.001) were independently associated with a better PFS.
Low dose apatinib was safe in HCC patients, with the most common adverse events being hypertension (29.2 %), fatigue (16.9 %), hand and foot syndrome (16.3 %) and vomiting (14.0 %). Very few grade ≥ 3 AEs were observed; they included decreased platelet, diarrhea, and bradycardia (1 patient each, 0.6 %).
The efficacy outcomes observed in this real-world analysis were significantly better with apatinib compared to clinical data obtained with sorafenib or lenvatinib. Therefore, low dose apatinib could provide a new treatment option for advanced HCC, with early application of apatinib and combination treatment potentially providing an even better efficacy.
Figure 2: Progression-free survival (A), overall survival (B) and response rate (C) for all patients treated with apatinib.
Sequential treatment with chemoembolization plus radiotherapy followed by immunotherapy
So far, HCC has successfully been managed by the means of therapeutic synergy between loco-regional therapies and checkpoint inhibitors [22, 23]. Avelumab is an immune checkpoint inhibitor that has shown clinical efficacy in several malignancies, including advanced renal cell carcinoma [24], advanced or metastatic urothelial carcinoma [25] and advanced HCC [26].
START-FIT is a single arm, phase II study (NCT03817736) that evaluated the safety and efficacy of sequential transarterial chemoembolization (TACE) and stereotactic body radiotherapy (SBRT), followed by avelumab, in patients with locally advanced HCC [27]. Eligible patients were not candidates for curative resections with locally advanced HCC of at least 5 cm, less than three tumor nodules and child-Pugh A5-B7 liver function. The primary endpoint was the percentage of patients amenable to curable surgery, while the secondary endpoints included ORR according to modified RECIST v1.1, OS and TRAEs. Patients enrolled in this study received a single episode of TACE followed by 5-fraction SBRT (28 days afterwards), followed by avelumab (10 mg/kg, 14 days afterwards and Q2W thereafter).
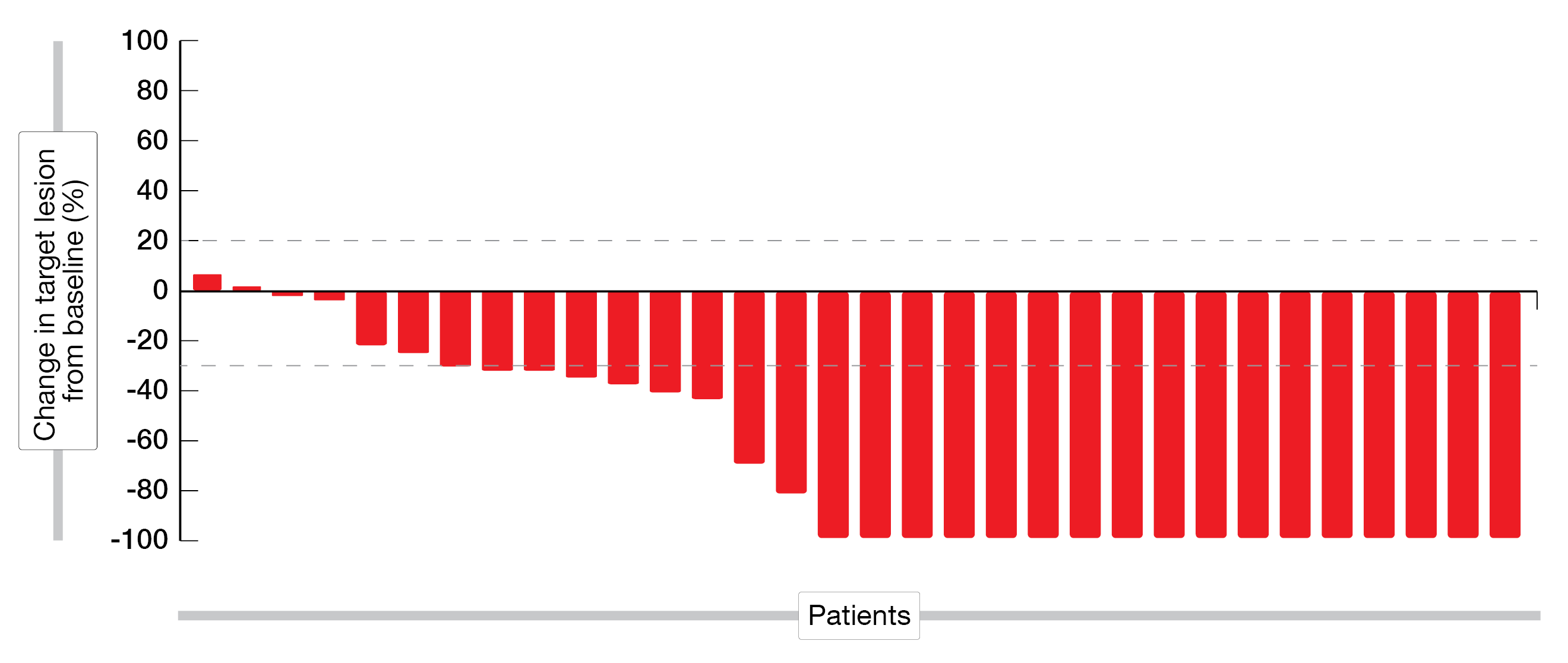
Among the 33 enrolled patients, the median age was 68 years (range, 51 – 81) with only one female patient being recruited. After a median follow-up of 17.2 months, the ORR reached 62.5 % (95 % CI, 45.3 – 77.1), including a CR rate of 43.8 % and a PR rate of 18.8 %. The median OS was 30.3 months (95 % CI, 22.7 – 37.8) and the median PFS was 20.7 months (95 % CI, 14.6 – 26.8). The outcome of this combined treatment on the size of the tumor lesions is shown in Figure 3.
In total, ten patients (30.3 %) experienced grade ≥ 3 TRAEs, the most common being a transient increase in ALT/AST (12.1 %) and an increased bilirubin level after TACE (6 %). Five patients (15.2 %) had grade ≥ 3 irAEs (hepatitis, n = 3; dermatitis, n = 2).
In total, only 9 % of the enrolled patients were downstaged to receive curative therapy; the combination of loco-regional treatment and immunotherapy resulted in an unexpected high cure rate of 43 % and a high overall survival rate in patients with locally advanced unresectable HCC.
Figure 3: Waterfall plot, change in sum of longest diameter of taArget lesions from baseline (%).
REFERENCES
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Finn RS et al., IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020; 382(20): 1894-1905.
- Cheng AL et al., Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25-34.
- Llovet JM et al., SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359(4): 378-390.
- Kudo M et al., Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391(10126): 1163-1173.
- Borbath Y et al., Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma (aHCC): 3-year follow-up of the phase 2 KEYNOTE-224 study. J Clin Oncol 2022; 40(suppl 16; Abstr 4109).
- Xing B et al., KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in combination with lenvatinib in the treatment for advanced unresectable or metastatic hepatocellular carcinoma (HCC): Preliminary efficacy and safety results of a prospective phase II trial. Ann Oncol 2021; 32(suppl 5; S822/938P).
- Xing B et al., A phase II study combining KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) and lenvatinib in the treatment for advanced unresectable or metastatic hepatocellular carcinoma (HCC): Updated efficacy and safety results. J Clin Oncol 2022; 40(suppl 16; Abstr 4115).
- Van Cutsem EK et al., Tislelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): impact on health-related quality of life. ESMO Open 2022; 7(4):100517.
- Lu S et al., Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol 2021; 16(9):1512-1522.
- Ren Z et al., Tislelizumab monotherapy for patients with previously treated advanced hepatocellular carcinoma (HCC): RATIONALE-208 Chinese subpopulation. An Oncol 2022; 33(suppl 4:S255/P-25).
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRs has a profound impact on its biological functions. Cancer Immunol Immunotherapy 2018; 67(7):1079-1090.
- Dahah R et al., FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis Cancer Cell 2015; 28:285-295.
- Edeline J et al., Clinical outcomes associated with tislelizumab in patients (pts) with advanced hepatocellular carcinoma (HCC) who have been previously treated with sorafenib (SOR) or lenvatinib (LEN) in RATIONALE-208. J Clin Oncol 2022; 40(suppl 16; Abstr 4072).
- Bruix J et al., Update on Medical Treatment for Advanced Hepatocellular Carcinoma. Lancet 2018; 389;58-88
- Finn RS et al., Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020; 382(20):1894-1905.
- Ostroumov D et al., Transcriptome Profiling Identifies TIGIT as a Marker of T-Cell Exhaustion in Liver Cancer. Hepatology 2021; 73:1399-1418.
- Siegel, AB et al., Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol 2008; 26:2992-2998.
- Fan J et al., AdvanTIG-206: Anti-TIGIT monoclonal antibody (mAb) ociperlimab (BGB-A1217;OCI) plus anti-programmed cell death protein-1 (PD-1) mAb tislelizumab (TIS) plus BAT1706 versus TIS plus BAT1706 as first-line (1L) treatment for advanced hepatocellular carcinoma (HCC). J Clin Oncol 2022; 40(suppl 16; Abstr TPS4172).
- Qin S et al., Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2021; 6(7):559-568.
- Meng L et al., Efficacy and safety of low-dose apatinib in advanced hepatocellular carcinoma. J Clin Oncol 2022; 40(suppl 16; Abstr 4104).
- Llovet JM et al., Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021; 18(5):293-313.
- Lee YH et al., Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front Immunol 2020; 11:568759.
- Choueiri TK et al., Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 2020; 31(8):1030-1039.
- Powles T et al., Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020; 383(13):1218-1230.
- Kudo M et al., Avelumab in Combination with Axitinib as First-Line Treatment in Patients with Advanced Hepatocellular Carcinoma: Results from the Phase 1b VEGF Liver 100 Trial. Liver Cancer 2021; 10(3):249-259.
- Chiang CL et al., Sequential trans-arterial chemoembolization and stereotactic body radiotherapy followed by immunotherapy (START-FIT) for locally advanced hepatocellular carcinoma: A single-arm, phase II trial. J Clin Oncol 2022; 40(suppl 16; Abstr 4091).
© 2022 Springer-Verlag GmbH, Impressum