Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year [1]. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months [2].
Tislelizumab as first-line treatment option
Tislelizumab is a humanized monoclonal antibody directed against programmed cell death protein 1 (PD-1), engineered to minimize binding to the Fc receptors for IgG (FcyR) on macrophages to evade antibody-dependent cellular phagocytosis, a mechanism of T-cell clearance and potential anti PD-1 resistance [3, 4]. Recent phase 2 and 3 studies have shown that tislelizumab was efficacious in the management of multiple solid tumor entities [5-8].
RATIONALE-309 is a randomized, double-blind phase 3 study (NCT03924986) which analyzed 263 patients with recurrent or metastatic NPC; those patients were randomly assigned 1:1 to receive tislelizumab (200 mg intravenously [IV]) or placebo on Day 1, plus gemcitabine (1 g/m2, IV, Day 1, Day 8), plus cisplatin (80 mg/m2, Day 1) every three weeks (Q3W) for 4–6 cycles, followed by tislelizumab or placebo Q3W until disease progression, unacceptable toxicity, or withdrawal. Patients in the placebo arm could crossover to tislelizumab monotherapy if disease progression was confirmed by the independent review committee (IRC) and the investigator considered it clinically beneficial. IRC-assessed PFS was the primary endpoint and IRC-assessed objective response rate (ORR), as well as duration of response (DoR), OS, investigator assessed PFS, time to second objective disease progression (PFS2) and safety were the secondary endpoints.
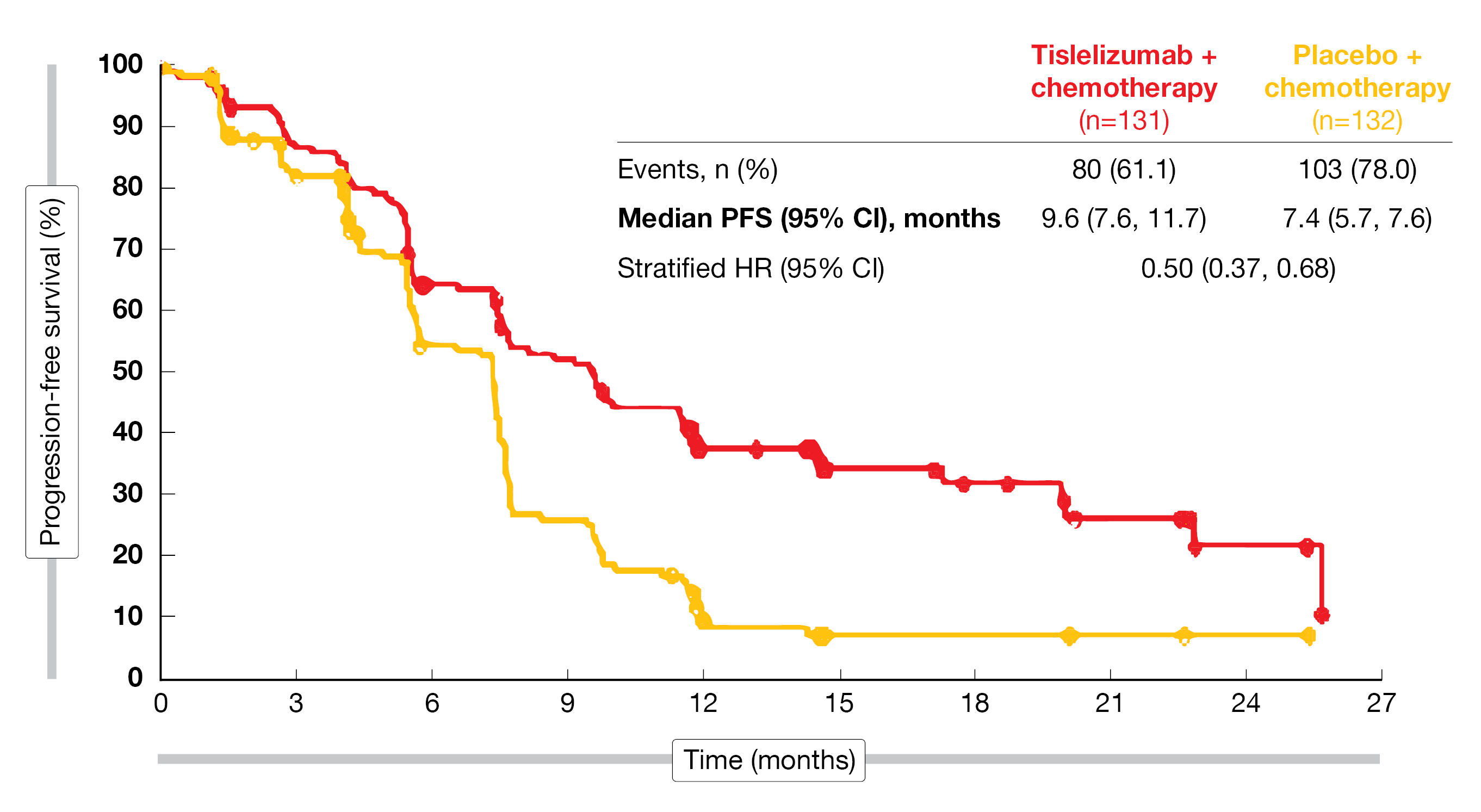
The results of RATIONALE-309 trial were consistent with interim data [9] and demonstrated a clinically meaningful improvement for tislelizumab plus chemotherapy versus placebo plus chemotherapy (mPFS, 9.6 vs. 7.4 months; HR, 0.50; 95 % CI, 0.37-0.68) after a median follow-up of 15.5 months (Figure 1) [10]. To note, the PFS benefit observed was independent of PD-L1 expression, as an improvement in PFS for tislelizumab plus chemotherapy versus placebo plus chemotherapy was observed in all subgroups (< or ≥ 1 % and < or ≥ 10 %). Additionally, a numerical OS benefit was observed in the investigational arm with mOS not yet reached in the tislelizumab combination arm and 23 months for the chemotherapy plus placebo arm (HR, 0.60; 95 % CI, 0.35-1.01). For patients treated with tislelizumab plus chemotherapy, median PFS2 was not yet reached compared to 13.9 months for those treated with placebo plus chemotherapy (HR, 0.38; 95 % CI, 0.25-0.58). Moreover, gene expression profiling identified three gene expression clusters (cold, medium, hot) as potential biomarkers for efficacy. A hot tumor immune profile, characterized by the highest expression of immune cells in the tumor microenvironment (including dendritic cells) was associated with a greater PFS advantage versus cold tumors for tislelizumab plus chemotherapy. The safety profile of tislelizumab plus chemotherapy was manageable and as expected based on previously reported interim analysis. The following treatment emergent adverse events (TEAEs) grade ≥3 were experienced by at least 20 % of patients: decrease in white blood cell count, anemia, decrease in neutrophil count, neutropenia, decrease in platelet count, and leukopenia.
In this study, the combination of tislelizumab and chemotherapy provides a consistent, clinically meaningful improvement in PFS, accompanied by PFS2 and OS benefits, compared with placebo plus chemotherapy. Thus, the authors concluded that this combined therapy has the potential to become a new standard-of-care 1L treatment for patients with recurrent or metastatic NPC.
Figure 1: Updated PFS analysis of tislelizumab plus chemotherapy versus placebo plus chemotherapy.
Anti-angiogenic therapy: synergistic activity with immune checkpoint inhibitors
In patients with recurrent or metastatic immune checkpoint inhibitor (ICI)-resistant nasopharyngeal cancer, ICI given in combination with an antiangiogenic therapy might lead to a potential synergistic effect [11]. Camrelizumab – a programmed cell death 1 (PD-1) inhibitor – has been investigated as treatment of various malignancies and has demonstrated a significantly improved OS or PFS when administered in combination with chemotherapy in phase 3 trials among patients with advanced or metastatic esophageal squamous cell carcinoma [12] or NPC [13]. Famitinib – a receptor tyrosine kinase inhibitor – showed a prolonged PFS in refractory patients with metastatic colorectal cancer when administered as monotherapy [14], and a potent and enduring antitumor activity when combined with camrelizumab in patients with advanced or metastatic renal cell carcinoma [15].
An open-label, multi-center, phase II basket trial (NCT04346381) evaluated the use of camrelizumab plus famitinib for the treatment of recurrent or metastatic, ICI-resistant NPC [16]. Patients who met inclusion criteria – histologically confirmed recurrent or metastatic NPC (nonkeratinizing carcinoma, WHO type II-III), who had been treated with platinum-based chemotherapy and ICIs (≤ 2 lines of systemic treatment) – were enrolled to receive camrelizumab (200 mg intravenously [IV] Q3W) and famitinib (20 mg orally, once daily). The primary endpoint was the ORR according to RECIST v1.1, while the DoR, disease control rate (DCR), time to response (TTR), PFS, OS and safety were secondarily analyzed.
Data reported at ASCO 2022 showed that of the 15 patients enrolled in this study, twelve (80 %) had received a prior 1L therapy and three (20 %) patients a second-line treatment. All patients were pretreated with anti-PD-1/PD-L1 immunotherapies (toripalimab, n = 9; tislelizumab, n = 4; sintilimab, n = 1; pembrolizumab, n = 1). After a median follow-up of 6.3 months, the ORR reached 33.3 % (95 % CI, 11.8-61.6), five patients had a confirmed partial response (PR), seven patients a stable disease (SD), two patients a progressive disease (PD) and one was not evaluated. The DCR was 80.0 % (95 % CI, 51.9-95.7), the median DoR was 4.2 months (95 % CI, 2.1-not reached [NR]), the median PFS was 6.3 months (95 % CI, 4.1-NR), while the median OS has not been reached.
The most common grade ≥ 3 treatment emergent adverse events (TEAEs) were a decreased platelet count, a decreased neutrophil count and a palmar-plantar erythrodysaesthesia syndrome (13.3 % each). In total, eleven patients had to interrupt, and one patient had to discontinue the treatment due to TRAEs. Moreover, five patients had serious TRAEs (grade 2 platelet count decreased, grade 2 pharyngeal necrosis, grade 2 pulmonary tuberculosis, grade 3 left ventricular dysfunction, grade 3 pharyngeal hemorrhage; 1 patient each).
In summary, the combination of camrelizumab plus famitinib, due to the encouraging antitumor activity, may be a novel efficient and safe alternative therapeutic approach for difficult to treat NPC and support further investigation.
Camrelizumab combined to apatinib
Apatinib is a VEGFR2 tyrosine kinase inhibitor, which has been shown to optimize the tumor immunosuppressive microenvironment and therefore to potentiate the antitumor effect of anti-PD-1/PD-L1 blockade in lung cancer [17]. Previous data demonstrated that apatinib has also proven clinical efficacy in recurrent or metastatic NPC [17, 18]. Moreover, apatinib combined with camrelizumab showed promising synergistic efficacy and manageable safety in patients with advanced HCC [19].
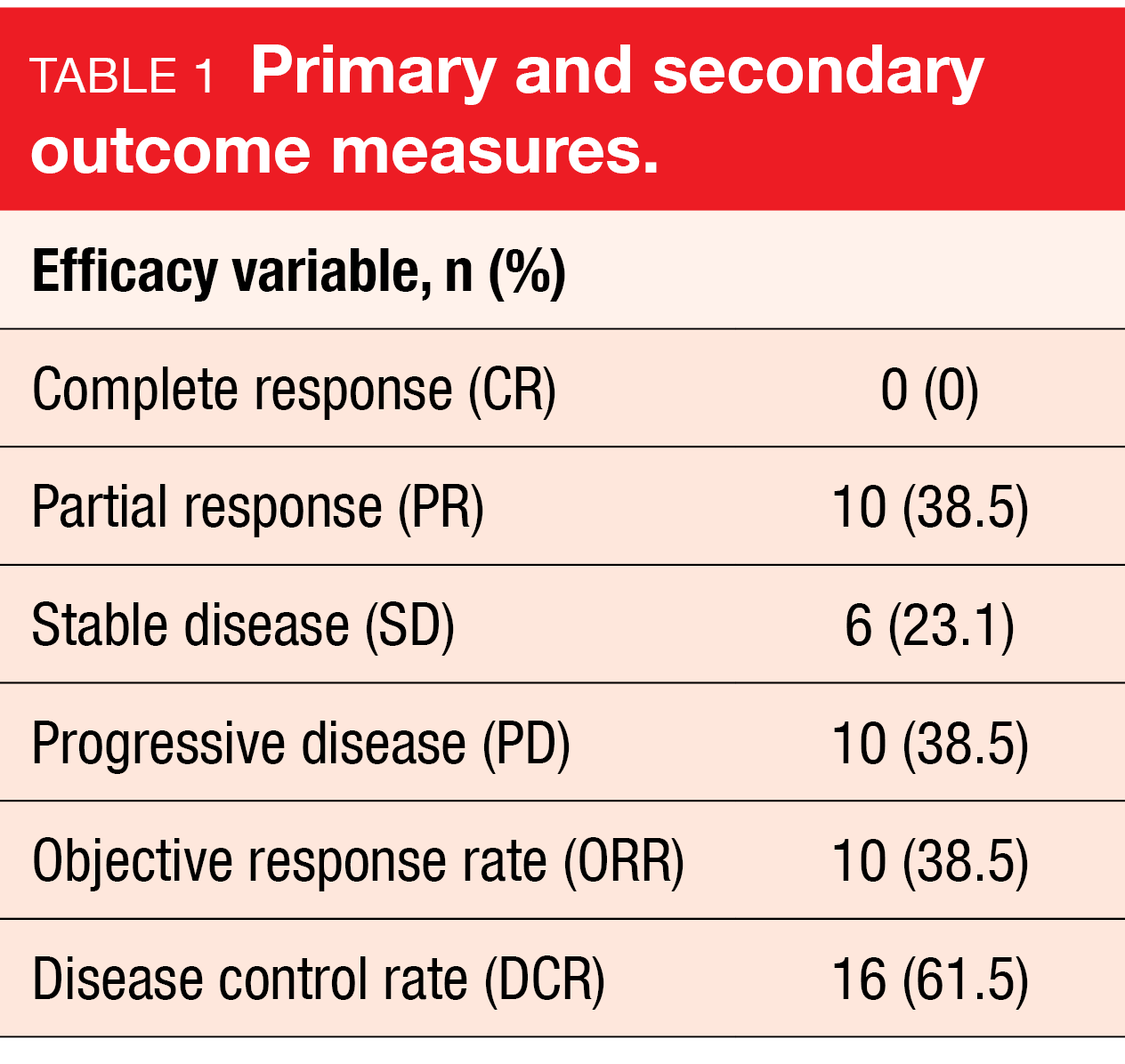
In the phase 2 study, 26 patients with recurrent or metastatic NPC were enrolled (between January 2011 and September 2021) to receive either apatinib (250 mg, orally once daily) plus camrelizumab (200 mg, IV, Q2W) until disease progression or unacceptable toxicity [20]. The median age was 49 years (range, 33-67), most of them were male (84.6 %), the most frequent sites of metastasis were the bone (53.8 %), the lung or the liver (38.5 % each).
After a median follow up of eight months, the ORR according to RECIST version 1.1. – the primary endpoint –reached 38.5 % (10/26) – with ten patients having a PR (38.5 %). The DCR was 61.5 % (16/26), the mPFS six months and mOS was not reached yet. TEAEs were in line with those expected: six patients had grade 3 or 4 TEAEs, including anemia (7.7 %), as well as stomatitis, headache, pneumonia and myocarditis (3.8 % each).
The authors concluded that camrelizumab plus apatinib had promising antitumor activity and manageable toxicities in this patient population. Hence, larger randomized trails are warranted to further evaluate this new combinational therapy.
Anlotinib: inhibition of tumor angiogenesis
A novel oral tyrosine kinase inhibitor, anlotinib, has been developed to inhibit tumor angiogenesis and proliferation by targeting vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit [21]. Anlotinib has already shown encouraging efficacy, as well as a manageable and tolerable safety profile, in a broad range of malignancies, including advanced non-small cell lung cancer [22] or sarcoma [23].
A recent prospective, single arm, phase 2 study (NCT03906058) assessed the efficacy and safety of the single agent anlotinib in patients with recurrent or metastatic NPC [24] with anlotinib being administered orally at 12 mg daily from Day 1 to Day 14 Q3W until disease progression or intolerable toxicity. Eligible patients were aged 18-70 years, had to present with a histologically confirmed recurrent or metastatic NPC, and at least one measurable lesion as well as at least two failed lines of prior systemic treatments (including chemotherapy, targeted therapy, or immunotherapy). Confirmed DCR was the primary endpoint and tumor response (confirmed ORR according to RECIST v1.1), PFS, OS and safety according to NCI-CTCAE v5.0 were the secondary endpoints.
Among 39 patients (84.6 % male) were enrolled in this study from April 2019 to March 2021; the mean age was 46.7 years (range, 20-64), 61.5 % of patients had liver metastasis, 74.4 % had previously received two lines of systemic treatments and 48.7 % had a prior anti-PD-1 immunotherapy. Anlotinib was given for a median of four cycles (range, 0.5-20). Of the 36 patients evaluated, the ORR was 22.2 % and the DCR 77.8 %, with a PR in eight patients and a SD in 20 patients. The mPFS was 5.7 months (95 % CI: 4.7-6.7) and the mOS was 23.9 months (95 % CI: 5.3-42.5).
TRAEs were manageable, with hand foot mouth syndrome (HFS) any grade occurring in 24 patients (61.5 %). Grade 3 or 4 TRAEs included hypertension (54 %), hand-foot skin reaction (23 %), mucositis (21 %), liver dysfunction (5 %) and pneumonia (3 %).
This initial data support anlotinib as a new monotherapy for patients with recurrent or metastatic NPC due to the observed survival benefit in this heavily treated population.
First-in-class bintrafusp alfa in previously treated patients
To improve the poor prognosis of patients with recurrent or metastatic NPC who progressed after platinum-based chemotherapy, the clinical activity and safety of bintrafusp alfa – a first-in-class bifunctional fusion protein that blocks PD-L1 and neutralizes TGF-α- was investigated. Bintrafusp alfa recently showed its ability to induce polyclonal neoadjuvant-specific T-cell responses in tumors in head and neck cancer [25].
A single arm, prospective phase 2 study (NCT04396886) evaluated the antitumoral activity of bintrafusp alfa in patients with heavily pretreated recurrent or metastatic NPC. Overall, 43 patients with recurrence at distant sites and not eligible for curative treatment were screened and 38 were enrolled in this trial [26]. NPC patients with histologically confirmed NPC, who had at least one prior line of platinum-based chemotherapy for recurrent disease, were subsequently treated with bintrafusp alfa (1200 mg, Q2W) until disease progression. Investigators set out to measure ORR as the primary endpoint, while survival and toxicity were the secondarily analyzed endpoints.
After a median follow-up of 14.9 months (range, 1.6 – 23.3), the confirmed ORR was 23.7 % (95 % CI, 12.4-38.8) including one patient with a CR and eight patients with a PR. In total, the median treatment duration was 1.8 months (range, 0.5-14.3 months). To note, eight patients (21.1 %) received bintrafusp alfa for more than six months and two patients (5.3 %) for more than twelve months. At Week 4, ORR was higher in patients with decreased EBV-DNA levels (40 vs. 6.3 %, p = 0.02), whereas high exosomal PD-L1 levels seemed to be predictive of worse ORR (5.3 vs. 41.7 %, p = 0.012). No associations were shown between clinical outcome and tissue PD-L1 expression or plasma TGF-β clearance. The 1-year OS rate reached 57.5 % (95 % CI, 40.2-71.5) and the 1-year PFS rate was 23 % (95 % CI, 10.1-39.4).
Grade ≥ 3 treatment-related adverse events (TRAEs) were observed in 16 patients and most commonly included anemia (23.7 %) and secondary malignancies (10.5 %).
The authors concluded that bintrafusp alfa has promising antitumor activity in heavily pretreated recurrent or metastatic NPC patients.
SHR-1701: Expanding the clinical benefit of immune checkpoint inhibitors
The antitumor activity of ICIs in recurrent or metastatic NPC was only seen in a subset of patients [13]. In an attempt to expand the clinical benefit of ICIs to more patients, ICIs were combined to agents that block immunosuppressive pathways, like TGF-β, and investigated in advanced solid tumors [27, 28]. The purpose of the NCT04282070 study was to evaluate the efficacy and safety of SHR-1701 – a bifunctional fusion protein composed of a monoclonal antibody against PD-L1 fused to the extracellular domain of the TGF-β receptor II – in patients with recurrent or metastatic NPC.
The ongoing multicenter, open-label, phase 1b study evaluates the safety and efficacy of SHR-1701 (30 mg/kg, Day 1) monotherapy, or in combination with cisplatin (80 mg/m2, Day 1) and gemcitabine (1000 mg/m2, Day 1 and Day 8), or in combination with albumin-paclitaxel (260 mg/m2, Day 1) in 3-week cycles in patients with recurrent or metastatic NPC until confirmed progression, unaccepted toxicity or patient withdrawal [29]. The primary endpoint for this study was safety. This study reports on an analysis of patients who failed previous platinum-based chemotherapy (Arm 1) or both platinum-based chemotherapy and anti-PD-1/PD-L1 antibody treatment (Arm 2). All NPC patients included in the analysis had stage IVb disease and metastatic lesions. The primary endpoint concerned safety, while the secondary endpoints included ORR, DoR, DCR, PFS and OS.
Among the 56 eligible patients who were enrolled (Arm 1, n = 30; Arm 2, n = 24), 51.8 % (Arm 1, n = 13; Arm 2, n=16) had received ≥ 2 prior lines of therapy. Grade ≥ 3 TRAEs occurred in ten patients (18.5 %), the most frequent being anemia (7.4 %) and hemoptysis (3.7 %). Only two patients (3.7 %) discontinued study treatment due to TRAEs (peripheral nerve injury and epistaxis, 1 patient each), ten patients (18.5 %) had dose delay because of AEs and Five patients (9.3 %) experienced grade ≥ 3 investigator reported immune related AEs (irAEs). Moreover, one death from unknown cause, not assessable SHR-1701, was reported.
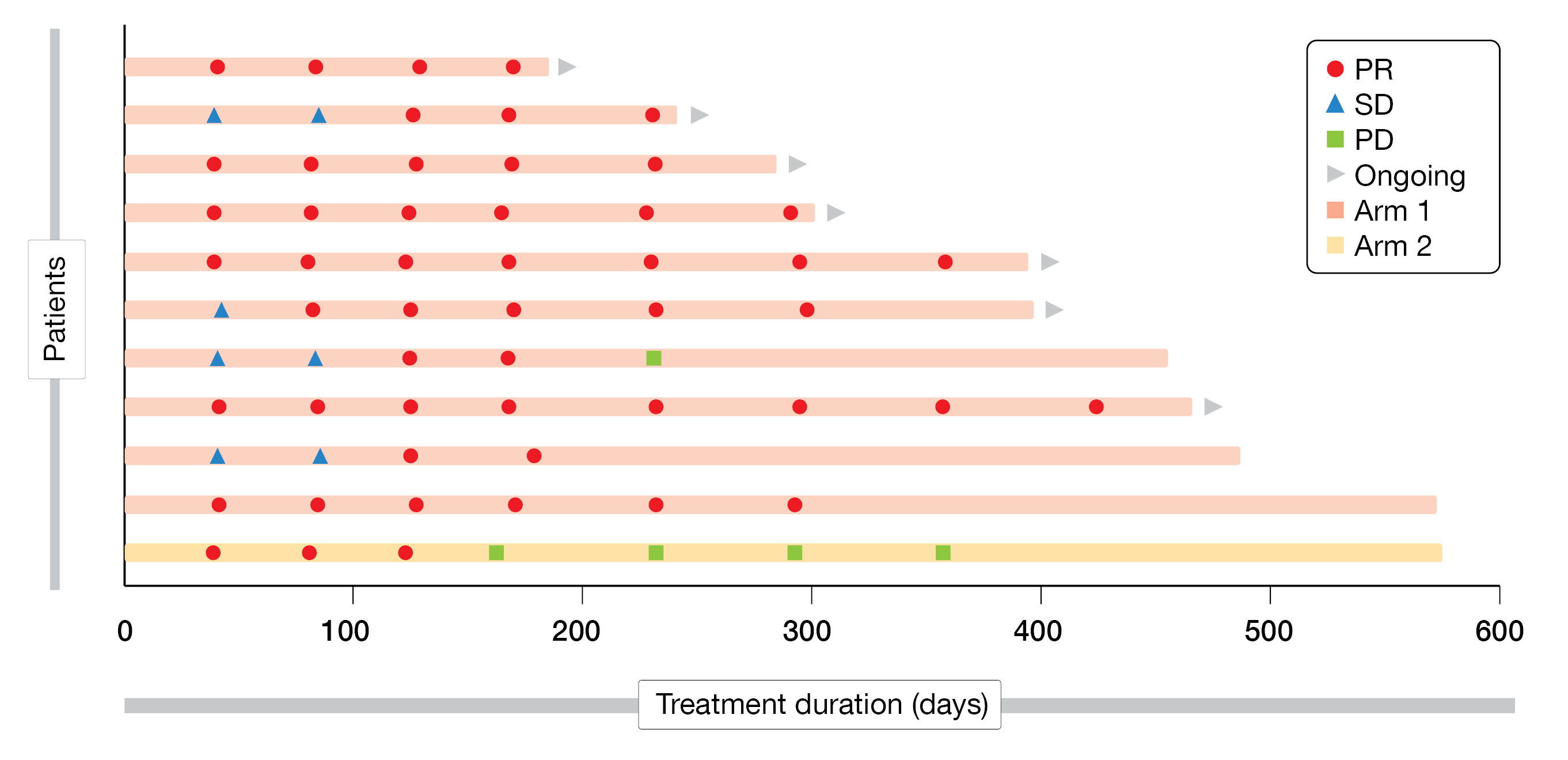
At data cutoff, the ORR reached 33.3 % (95 % CI, 17.3-52.8) in Arm 1 and 4.2 % (95 % CI, 0.1-21.1) in Arm 2, while the DCR was 53.3 % (95 % CI, 34.3-71.7) and 25.0 % (95 % CI, 9.8-46.7), respectively. Response was still ongoing in nine patients (Arm 1) and none in Arm 2 (Figure 2). The median DoR was not reached (Arm 1) or 4.1 months (Arm 2), while the median PFS reached 5.3 months (95 % CI, 1.3-not reached) in Arm 1 and 1.4 months (95 % CI, 1.3-2.7) in Arm 2. The median OS was not reached in both arms, but the 12-month OS rate was 79.9 % (95 % CI, 53.2-92.3) and 71.9 % (95 % CI, 47.6-86.4), respectively.
Overall, SHR-1701 showed a tolerable safety profile combined with good efficacy, leading the authors to conclude that it is a promising new antitumor treatment for patients with recurrent or metastatic NPC who have failed prior platinum-
based chemotherapy.
Figure 2: Treatment duration and response.
Dual immune checkpoint blockade for advanced NPC
Combination of anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibody and anti-programmed cell death 1 (PD-1) antibody is suggested to have a synergistic anti-tumor effect [30]. QL1706, a novel dual immune checkpoint blockade contains a mixture of anti-PD-1 IgG4 and anti-CTLA4 IgG1 antibodies produced by a single cell line. In the phase 1a dose escalation and expansion study (NCT04296994), patients received intravenous QL1706 at 0.3, 1.0, 3.0, 5.0, 7.5, or 10.0 mg/kg Q3W for dose escalation in an accelerated 3+3 design, whereas the dose expansion cohorts received selected doses. The aim was to define the safety, tolerability, and recommended phase 2 dose of QL1706. In phase 1b (NCT05171790), patients with advanced solid tumors were given intravenous QL1706 (5.0 mg/kg, Q3W), according to the data obtained in phase 1a, to evaluate the preliminary efficacy. Pooled analyses were conducted in the NPC cohorts receiving QL1706 (5 mg/kg). Additionally, dynamic changes of plasma EBV DNA level from baseline were determined in a part of patients during the studies [31].
As of Dec 31, 2021, a total of 110 patients with NPC were included of whom 79 (71.8 %) patients had ≥ two prior treatment lines and 48 (43.6 %) patients received previous immunotherapy. After a median follow-up of 7.7 months, confirmed overall response was reached in 27 patients (24.5 %; 95 % CI, 16.8-33.7). In immunotherapy-naive patients with one and ≥ 2 prior lines of treatment, ORR were 39.1 % (9/23) and 38.5 % (15/39), respectively. Three of 48 (6.3 %) immunotherapy-treated patients had partial response. Disease control was observed in 54 (49.1 %; 95 % CI, 39.4-58.8) patients. Median DoR reached 11.7 months (95 % CI 8.1-not estimable). Median PFS was 2.0 months (95 % CI 1.4-2.9) and overall survival data were immature. Patients with ≥ 50 % decrease in EBV DNA level on Day 43 had significantly better ORR than those with < 50 % decrease (67 % [8/12] versus 12 % [2/17]; p = 0.0045). TRAEs were reported in 85 (77.3 %) patients. In total, 14 patients (12.7 %) experienced grade ≥ 3 TRAEs. The most common TRAEs were rash, hypothyroidism (25 [22.7 %], each), and pruritus (22 [20 %]). TRAEs leading to dose interruptions occurred in 10 (9.1 %) patients. No TRAE leading to dose discontinuation or death was reported. The immune-related TRAEs and serious TRAEs were observed in 51 (46.4 %) and eight patients (7.3 %), respectively.
Based on the impressive anti-tumor effects of QL1706 on advanced NPC, accompanied by acceptable tolerability and manageable toxicity, further investigation of QL1706 in NPC is continuing.
REFERENCES
- Global Cancer Observatory. Cancer Today. Lyon France International Agency for Research on Cancer. Last assessed on June 20, 2022; Available from: https://gco.iarc.fr/today/data/factsheets/cancers/4-Nasopharynx-fact-sheet.pdf.
- Zhang, L, et al., Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016; 388(10054): 1883-1892.
- Zhang, T, et al., The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Dahan, R, et al., FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015; 28(3): 285-95.
- Ye, D, et al., Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci 2021; 112(1): 305-313.
- Shen, L, et al., Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8(1).
- Lu, S, et al., Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol 2021; 16(9): 1512-1522.
- Wang, J, et al., Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021; 7(5): 709-717.
- Yang, Y, et al., 121O RATIONALE 309: A randomized, global, double-blind, phase III trial of tislelizumab (TIS) vs placebo, plus gemcitabine + cisplatin (GP), as first-line treatment for recurrent/metastatic nasopharyngeal cancer (RM-NPC). Annals of Oncology 2021; 32: S1430.
- Zhang, L, et al., RATIONALE-309: Updated progression-free survival (PFS), PFS after next line of treatment, and overall survival from a phase 3 double-blind trial of tislelizumab versus placebo, plus chemotherapy, as first-line treatment for recurrent/metastatic nasopharyngeal cancer. J Clin Oncol 2022; 40(suppl 36; Abs 384950).
- Song, Y, et al., Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020; 11: 1956.
- Luo, H, et al., Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. Jama 2021; 326(10): 916-925.
- Yang, Y, et al., Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2021; 22(8): 1162-1174.
- Xu, RH, et al., Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer 2017; 36(1): 97.
- Qu, YY, et al., Camrelizumab plus Famitinib in Patients with Advanced or Metastatic Renal Cell Carcinoma: Data from an Open-label, Multicenter Phase II Basket Study. Clin Cancer Res 2021; 27(21): 5838-5846.
- Chen, M-Y, et al., Camrelizumab plus famitinib in patients with recurrent or metastatic nasopharyngeal carcinoma: Data from an open-label, multicenter phase II basket study. J Clin Oncol 2022; 40(suppl 16; Abs e18031).
- Zhao, S, et al., Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019; 7(4): 630-643.
- Ruan, X, et al., Apatinib for the treatment of metastatic or locoregionally recurrent nasopharyngeal carcinoma after failure of chemotherapy: A multicenter, single-arm, prospective phase 2 study. Cancer 2021; 127(17): 3163-3171.
- Xu, J, et al., Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res 2021; 27(4): 1003-1011.
- Mo, Y, et al., Camrelizumab plus apatinib in patients with recurrent and/or metastatic nasopharyngeal carcinoma: A single-arm, multicenter, phase 2 trail. J Clin Oncol 2022; 40(suppl 16; Abs e18025).
- Gao, Y, et al., Anlotinib as a molecular targeted therapy for tumors. Oncol Lett 2020; 20(2): 1001-1014.
- Zha, B, et al., Efficacy and safety of anlotinib as a third-line treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Oncol Lett 2022; 24(1): 229.
- Yao, W, et al., Long-Term Efficacy and Safety of Anlotinib as a Monotherapy and Combined Therapy for Advanced Sarcoma. Onco Targets Ther 2022; 15: 669-679.
- Cai, Q, et al., Anlotinib for patients with recurrent or metastatic nasopharyngeal carcinoma: A phase II study. J Clin Oncol 2022; 40(suppl 16; Abs e18020).
- Redman, JM, et al., Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-b blockade in HPV-unrelated head and neck cancer. J Clin Invest 2022.
- Chiang, C, et al., Antitumor activity of bintrafusp alfa in previously treated patients with recurrent or metastatic nasopharyngeal cancer (NPC): A single arm, prospective phase II trial. J Clin Oncol 2022; 40(suppl 16; Abst e18029).
- Lan, Y, et al., Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 2018; 10(424).
- Strauss, J, et al., Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res 2018; 24(6): 1287-1295.
- Yang, Y, et al., A phase Ib study of SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with recurrent or metastatic nasopharyngeal carcinoma (RM-NPC). J Clin Oncol 2022; 40(suppl16; Abst 6024).
- Rotte A., Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255.
- Zhao, H, et al., Efficacy and safety of QL1706, a novel dual immune checkpoint blockade containing a mixture of anti-PD1 IgG4 and anti-CTLA4 IgG1 antibodies, for advanced nasopharyngeal carcinoma (NPC): Pooled cohort data from phase 1a/1b trials. J Clin Oncol 2022; 40(suppl16; Abst 6034).
© 2022 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
New therapeutic options being currently investigated in advanced or metastatic colo
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
An update and future directions in advanced gastric or gastrointestinal junction ca
Innovative combinations in esophageal squamous cell carcinoma
Innovative combinations in esophageal squamous cell carcinoma Each year, esopha
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer N
Preface ASCO Solid Tumor 2022
Preface – ASCO Solid Tumor 2022 © Private – Keun-Wook Lee, MD, PhD,