Further benefits of PET imaging in prostate cancer
Prostate cancer was the most frequently diagnosed cancer in men in 112 countries around the world in 2020, with 1.4 million newly diagnosed cases leading to more than 375,000 deaths [1].
VISION: association between baseline PSMA-PET scans and clinical outcomes in patients with mCRPC
The open-label, multi-center, randomized phase 3 VISION study (NCT03511664) demonstrated the efficacy and safety of 177Lu-PSMA-617 targeted radioligand therapy in patients with progressive prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) [2]. These positive results led to FDA approval and breakthrough therapy designation for 177Lu-PSMA-617 in later lines of mCRPC treatment [3,4]. Additionally, at last year’s EANM, Herrmann K. et al. presented data from the VISION study on health-related quality of life, pain, and safety [5]. All patients in VISION had to have PSMA-positive mCRPC, based on visual assessment of baseline gallium-68-radiolabelled prostate-specific membrane antigen 11 (68Ga-PSMA-11) PET/CT scans.
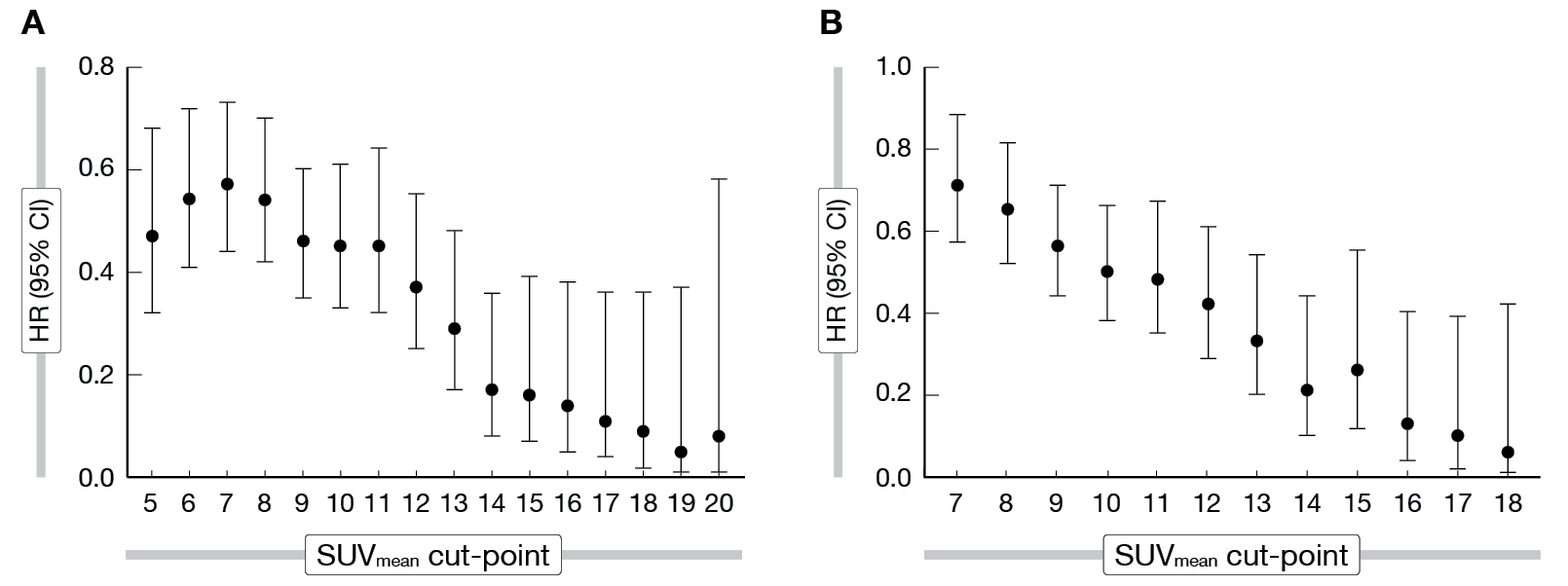
At the EANM 2023 congress, Phillip H. Kuo presented an exploratory post hoc analysis that examined the association between quantitative parameters from baseline 68Ga-PSMA-11 PET scans and the response to 177Lu-PSMA-617 in VISION [6]. The endpoints analyzed were radiographic progression-free survival (rPFS), overall survival (OS), objective response rate (ORR) and prostate specific antigen (PSA) response. The 831 patients were randomized 1:1 to 177Lu-PSMA-617 plus standard of care (SoC) or SoC only. Four quantitative 68Ga-PSMA-11 PET parameters from 826 patients were extracted: mean and maximum standardized uptake value (SUVmean and SUVmax) and tumor volume and load in bone, lymph node, liver, soft tissue, and the whole body. Higher SUV indicates higher PSMA expression, which may increase 177Lu-PSMA-617 tumor dose uptake, enhance anti-tumor activity, and lead to improved outcomes.
Baseline 68Ga-PSMA-11 whole-body SUVmean was the best predictor of outcomes in treatment-adjusted multivariate modeling. A 1-unit increase in whole-body tumor SUVmean was associated with a 12 % decrease in the risk of an rPFS event and a 10 % decrease in the risk of death. Compared with SoC alone, 177Lu-PSMA-617 improved outcomes across all whole-body SUVmean quartiles, but with greater benefits in patients with higher uptake values. In the highest whole-body SUVmean quartile (rPFS, ≥ 10.2; OS, ≥ 9.9) median rPFS was 14.1 months versus 3.9 months (HR, 0.34; 95 % CI: 0.20-0.56) and median OS was 21.4 months vs 15.0 months (HR, 0.47; 95 % CI: 0.32-0.68). In the lowest quartile (rPFS, < 6.0; OS, < 5.7), median rPFS was 5.8 months versus 3.9 months (HR, 0.75; 95% CI: 0.45-1.26) and median OS was 14.5 months versus 11.3 months (HR, 0.87; 95 % CI: 0.60-1.27). However, no SUVmean cut-point was better than any other for dividing patients receiving 177Lu-PSMA-617 into subgroups with longer or shorter rPFS and OS. Outcomes improved continuously with each unit increase in whole-body tumor SUVmean (Figure 1). Additionally, in both study groups, shorter rPFS and OS were associated with higher whole-body tumor load and the presence of lesions in bone or liver on 68Ga-PSMA-11 PET.
The VISION study was not powered for PET subgroup analysis and PET scanners as well as parameters differed among sites. Despite these limitations, high whole-body tumor 68Ga-PSMA-11 uptake at baseline emerged as the strongest predictor of 177Lu-PSMA-617 efficacy in patients with PSMA-positive mCRPC, although efficacy was evident at all uptake levels in VISION. These results show that study eligibility criterion based visual assessment of 68Ga-PSMA-11 PET/CT scans allows selection of patients with a range of whole-body tumor radiotracer uptake levels, but who are suitable candidates for 177Lu-PSMA-617 targeted radioligand therapy.
Figure 1: 68Ga-PSMA-11 whole-body tumor SUVmean cut-point analysis within the 177Lu-PSMA-617 arm of VISION for (A) rPFS and (B) OS.
LuTectomy: 177Lu-PSMA-617 prior to radical prostatectomy in men with high-risk localized prostate cancer
High-risk localized prostate cancer (HRCaP) is associated with a high probability of local and systemic recurrence [7]. LuTectomy is a phase 1/2 study (NCT04430192) in patients with HRCaP who are eligible for radical prostatectomy (RP). It evaluates the dosimetry, efficacy, and toxicity of lutetium (177Lu) vipivotide tetraxetan – so called 177Lu-PSMA-617 or LuPSMA –, whose beneficial effect have already been demonstrated in patients with metastatic castration-resistant prostate cancer [8]. The primary objective of this study is to determine the radiation dose absorbed by the tumor and involved lymph nodes. First results were presented by Michael S. Hofman at EANM 2023 [9].
Patients with HRCaP (prostate specific antigen, PSA > 20 ng/mL, ISUP grade group 3-5, status ≥ cT2c or N1), high tumor uptake (SUVmax ≥ 20) on 68Ga-PSMA-11 PET/CT and scheduled for RP were included in this study. Cohort A (n = 10) received one cycle of 5 GBq LuPSMA and Cohort B (n = 10) received two cycles of 5 GBq LuPSMA (6 weeks apart). The radiation dose was estimated by SPECT/CT at 4-, 24- and 96-hours post-therapy. Safety assessments and RP surgery were scheduled six weeks after LuPSMA treatment.
Twenty patients with a median age of 66 years were enrolled; 30 % of patients (6/20) had N1 disease. The median PSA level was 18 ng/mL (IQR, 11-35) and the median PSMA-PET SUVmax was 31 (IQR, 26-36) at baseline. The highest tumor absorbed dose after Cycle 1 was in median 35.5 Gy (IQR, 19.5-50.1) for all lesions, 19.6 Gy (IQR, 11.3-48.4) for the prostate and 37.9 Gy (IQR, 33.1-50.1) for lymph nodes.
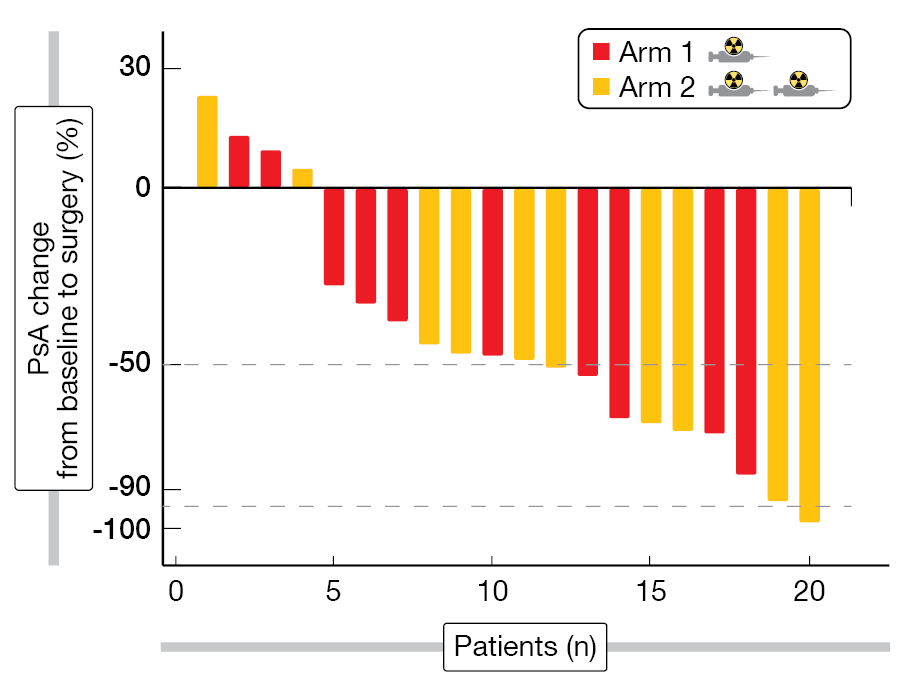
All patients underwent robotic RP, with surgical difficulty levels as expected in most patients (15/20) and slightly higher in five of them. After histopathologic analyses, 80 % of patients (16/20) patients showed a partial response and one patient had minimal residual disease, while no complete responses were obtained. The imaging response showed a stable SUVmax in 40 % of patients (8/20) and ≥ 30 % decrease in 55 % patients (11/20), while the rest presented with an SUVmax increase of more than 30 %. PSA was significantly reduced by 49 % (median, IQR, 32-67), with a reduction rate of at least 50 % in 45 % of patients (9/20) (Figure 2). After a median follow-up of 13.8 months, the biochemical recurrence-free survival reached 80 %.
Patients reported the following grade 1 adverse events (AEs), fatigue (40 %), nausea (35 %), dry mouth (30 %), thrombocytopenia (20 %) and lymphopenia (20 %); renal impairment of grade 2 was reported in one patient, while no grade 3/4 serious AEs (SAEs) occurred.
Overall, the authors concluded that 177Lu-PSMA-617 plus radical prostatectomy is safe in men with HRCaP with encouraging responses. Thus, further research is justified.
Figure 2: PSA change from baseline to surgery in patients having received one (red bars) or two (yellow bars) cycles of LuPSMA.
PSMA-PET/CT after salvage radiotherapy for recurrent or persistent prostate cancer after surgery
PSMA-PET is increasingly used to guide salvage radiotherapy (sRT) following radical prostatectomy in patients with recurrent or persistent prostate cancer [10]. In prostate cancer patients with biochemical recurrence after radical prostatectomy, PSMA-PET should be the tracer of choice when PET-CT imaging is considered for subsequent treatment management decisions [11]. In this context, sites of recurrence were clarified by PSMA-PET and disease localization translated into modified treatment in 68 % of patients with biochemical recurrence of prostate cancer [12]. Constantinos Zamboglou and colleagues investigated whether the implementation of PSMA-PET imaging improved the outcome after prostate cancer sRT in recurrent or persistent prostate cancer patients after surgery; these data were presented at EANM 2023 [13].
Patients having underwent sRT were selected from two databases: i) 344 patients from the multicenter phase III SAKK 09/10 trial (28 centers, 3 countries, non-PSMA-guided sRT for cMO); and ii) 1,548 patients from a retrospective multicenter cohort (11 hospitals, 5 countries, PSMA-guided sRT for cMO). Patients with positive lymph nodes in primary surgery, cM1 status (initial or visible in PET imaging), PSA > 0.5 ng/mL before sRT or missing stratification variables were excluded. Moreover, the cohorts were balanced based on age, ISUP score, PSA before sRT, T-stage and PSA recurrence versus persistence. The biochemical recurrence-free survival (BRFS) and recurrence patterns were compared, with biochemical recurrence defined as PSA nadir after sRT + 0.2 ng/mL.
A total of 717 patients were included in two well-balanced cohorts: Cohort 1 consisted in 255 patients (median follow-up of 75 months) and Cohort 2 in 462 patients (median follow-up of 36 months). In Cohort 2, local recurrence occurred in 33.8 % of patients and nodal recurrence in 22.3 %, associated to irradiation of lymph nodes in 22.3 % of patients.
The 3-year BRFS rates were 70 % (95 % CI: 64-77) and 78 % (95 % CI: 73-83) for Cohort 1 and Cohort 2 (p = 0.012, weighted log-rank test). This significant improvement in BRFS when PSMA-PET was used for sRT guidance can be explained by individualized sRT field based on PET. Thus, a consortium for further validation based on prospective data has been created: Co-IMPACT (Consortium for Implementation of PSMA-PET in Prostate Cancer Radiotherapy Trials) currently including approx. 7,000 patients from 13 countries in 38 centers dispatched in four cohorts. Co-IMPACT 1 will consist of 2,000 patients with primary prostate cancer, Co-IMPACT 2 will include 3,500 patients with recurrent prostate cancer after surgery and Co-IMPACT 4 will include 1,500 patients with recurrent prostate cancer after radiotherapy. CO-IMPACT 4 will assess the outcomes after PSMA-PET based radiotherapy in the castration-resistant setting.
REFERENCES
- Sung H et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3): 209-249
- Kuo PH et al. Why we did what we did: PSMA PET/CT selection criteria for the VISION trial. J Nucl Med. 2022; 63(6): 816-818
- Hofman MS et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018; 19(6):825-8 33
- Sartor O et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12): 1091-1103
- Hermann K et al. Health-related quality of life (HRQoL), pain and safety outcomes in the phase 3 VISION study of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. EANM 2022 (Oral abstract OP-161)
- Kuo PH et al. Association of baseline quantitative [68Ga]Ga-PSMA-11 PET imaging parameters with clinical outcomes in patients with mCRPC receiving [177Lu]Lu-PSMA-617: a VISION sub-study. EANM 2023 (Oral abstract OP-340).
- Reina Y et al. Advances in high-risk localized prostate cancer: Staging and management. Curr Probl Cancer. 2023; 47(4): 100993
- Dhiantravan N et al. Clinical trial protocol for LuTectomy: a single-arm study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 prior to prostatectomy. Eur Urol Focus. 2021; 7(2): 234-237
- Hofman M et al. LuTectomy: phase 1/2 study evaluating dosimetry, safety and potential benefit of pre-surgery [177Lu]Lu-PSMA-617 radioligand therapy in patients with high-risk localized prostate cancer. EANM 2023 (Oral abstract OP-338).
- Zamboglou C et al. Development and Validation of a Multi-institutional Nomogram of Outcomes for PSMA-PET-Based Salvage Radiotherapy for Recurrent Prostate Cancer. JAMA Netw Open. 2023; 6(5): e2314748
- Calais J et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019; 20(9): 1286-1294
- Fendler WP et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J Nucl Med. 2020; 61(12): 1793-1799
- Zamboglou C et al. Implementation of PSMA-PET/CT improves treatment outcomes after salvage radiotherapy for recurrent or persistent prostate cancer after surgery. EANM 2023 (Oral abstract OP-337).
© 2023 Springer-Verlag GmbH, Impressum
More posts
Theranostics: recent developments in neuroendocrine tumors
Theranostics: recent developments in neuroendocrine tumors 225Ac-DOTATATE daughter nuc