Recent progress in the treatment of neuroendocrine tumors
Combined radioligand and CAPTEM therapy in patients with advanced, non-resectable, progressive GEP-NET
Capecitabine plus temozolomide (CAPTEM) is widely used for the treatment of advanced, unresectable, and progressive neuroendocrine tumors (NETs). However, data are limited regarding its association with radioligand therapy (RLT)/peptide receptor radionuclide therapy (PRRT) [1-3]. PRRT is a radionuclide-targeted therapy that is based on the intravenous administration of radiolabeled somatostatin analogs that selectively target SSTR expressing cells [4]. At EANM 2023, Jarosław B. Ćwikła presented results of a prospective, single-arm, open-label, case series study (NCT04194125) that assessed the efficacy and safety of RLT and CAPTEM in patients with progressive advanced non-resectable gastroenteropancreatic neuroendocrine tumors (GEP-NETs), so called pancreatic (panNET) and midgut NETs [5]. The primary endpoint was the locally assessed progression free survival (PFS) according to RECIST v1.1. Secondary endpoints included the overall survival (OS), the objective response rate (ORR), the best objective response rate (BORR), the disease control rate (DCR), the clinical response based on a potential improvement of the performance status (PS – ECOG), and safety.
Out of 23 screened patients 21 were enrolled in the study (14 PanNET, 7 Midgut). All patients received RLT/PRRT (3 to 4 cycles of [177Lu]177LuDOTA-TOC of 5.55 - 7.4 GBq at each treatment session) at 8 to 12 weekly intervals, and concomitant CAPTEM. One patient received two treatment sessions, only. At baseline, the mean age was 58.6 years and 62 % of patients were women. Overall, seven patients presented with a NET grade 1, ten with a NET grade 2 and three with a NET grade 3 tumor. Eighty-six percent of tumors were classified as clinical stage IV, the rest was clinical stage III.
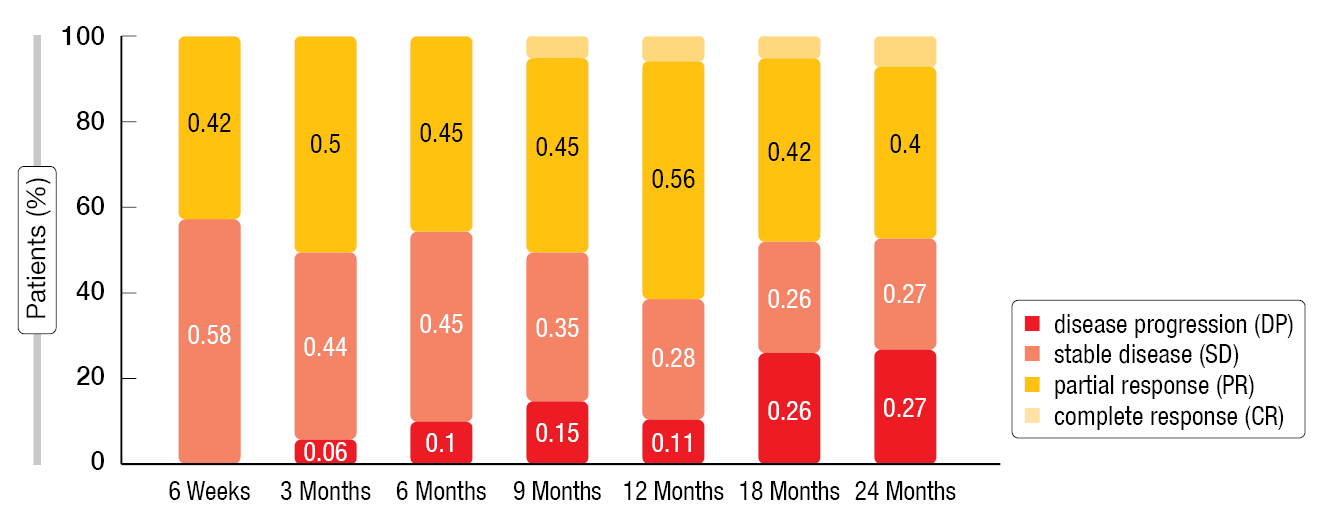
After a follow-up of at least 48 months, the median PFS was 31.5 months (IQR, 16.0-not reported [nr]) for the overall population (n=21). In the PanNET group, the mPFS was 28.0 months (IQR, 14.0-nr) and in the Midgut group 32.0 months (IQR, 24.5-nr), respectively. The median OS had not been reached yet. In terms of the ORR a partial response (PR) was reported in nine patients (42 %), while eleven patients (52 %) exhibited stable disease (SD) after six weeks of follow-up. At Month 12, one patient had a complete response (CR), ten had a PR (56 %), five a SD (26 %) and two a disease progression (DP) (11 %) (Figure 1). The DCR was 90 % at Week 6 and still 73 % after two years of follow-up. Moreover, the ECOG score of most patients improved.
The most frequently occurring adverse event (AE) related to the treatment was a transient hematological suppression. During the follow-up period, the most common AE was temporary lymphopenia, with 44 % classified as grade 2, 8 % as grade 3, and 2 % as grade 4. In total, 7 % of patients experienced thrombopenia. Other grade ≥3 AEs, such as increased gamma-glutamyl transferase [GGT], thrombocytopenia and anemia were observed sporadically. Notably, there were no other grade 4 AE reported either during or after the therapy.
Overall, the combined therapy based on RLT/PRRT and CAPTEM was efficient and well tolerated in most cases in patients with gastroenteropancreatic neuroendocrine (GEP-NETs) tumors (PanNET and Midgut), whereas a significant benefit in terms of ORR was reported in panNET patients only.
Figure 1: Objective response rate of the overall population (n = 21) from 6 weeks to 24 months according to RECIST v1.1 criteria.
177Lu-DOTATATE in BP-NENs: results of SEPTRALU registry
The efficacy and safety of 177LU-DOTATATE therapy (PRRT) in patients with advanced midgut grade 1/2 SSTR-positive NENs progressing to long-acting octreotide has already been shown in the phase 3 NETTER-1 trial [6]. Based on these data, it was assumed that PRRT could be effective in other tumors expressing SSTR, such as bronchopulmonary-NENs (BP-NENs). At this year’s EANM, Mercedes Mitjavila Casanovas presented an analysis conducted on patients from the Spanish multicenter (23 centers) SEPTRALU registry. Patients included in this analysis had advanced, non-resectable BP-NENs and had undergone treatment with PRRT between 2014 and 2022 (NCT04949282) [7, 8]. The dataset included treatments prior to PRRT, number of PRRT-cycles per patient, response to treatment, and disease progression. The response rate was assessed according to RECIST v1.1 criteria and the safety was evaluated with the Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Of the 706 NEN-patients included in this registry 9.35 % (n=66) had BP-NENs, with a median age of 62 years and 78 % of them were women. At the time of diagnosis, 84 % of patients had a performance status (PS – ECOG) of 0 or 1. Atypical carcinoid was the most common histological subtype found in 53 % of patients, followed by typical carcinoid (31 %) and NE carcinomas (16 %). Metastases were mainly located in the liver (80 %), lymph nodes (53 %) and bones (48 %). Most patients had received prior treatments before undergoing PRRT: 54 % underwent surgery, somatostatin analogs were administered to 92 %, and 43 % received everolimus. Overall, PRRT served as the second-line treatment for 31 % of patients, the third-line treatment for 58 % and was given after the third line in 12 % of cases, respectively. On average, the median duration from diagnosis to the initiation of PRRT was 35 months (95 % CI, 3-52).
The DCR in this study was 86 %, with 34 % of patients having a response (ORR=CR + PR, n = 22) and 52 % a SD (n = 34). In contrast, other studies reported a median ORR of 30 % (15-80 %) and a median DCR of 68 % (61-100 %) [9]. These differences in results might be attributed to the heterogeneity of the studied population, the relatively small sample size and variations in the criteria used for response analysis [8]. After a median follow-up of 31 months, the median PFS reached 18.4 months (95 % CI: 15.8-33.4) and the median OS was 47.9 months (95 % CI: 20-not applicable [NA]). This result is in line with previously published data (mPFS, 18.5 months; mOS, 48.6 months) in patients with typical/atypical lung carcinoids [9], but the group presented also includes patients with NE carcinoma. Of note, mPFS and mOS were shorter in PRRT-treated patients with BP-NETs compared to other NEN-sites [7, 9].
Grade 1-2 toxicities included neutropenia (44 %), nausea (40 %), and emesis (25 %). Seven patients (10 %) experienced grade 3 or 4 AEs, mostly hematological AEs (5.8 % of patients, no specific treatment was required) and nausea (1.5 %).
Overall, 177Lu-DOTATATE was shown to be an efficient and well tolerated therapeutic option in patients with advanced BP-NENs further supporting phase 3 studies.
Personalized 177Lu-DOTATATE PRRT of NETs
PRRT is widely administered with a fixed injected activity per cycle, leading to a high interpatient variability in healthy tissue dosimetry and a risk of under-treatment [10]. In this context, the updated efficacy and safety results of a prospective, open-label, single-center, phase 2 study (NCT02754297) were presented by Marc-André Morin at EANM 2023. In this trial a personalized PRRT-regime with a tailored 177Lu-DOTATATE activity was used to deliver a prescribed renal absorbed dose in PRRT-naïve patients with inoperable progressive and/or symptomatic NETs overexpressing SSTRs [10, 11].
Eligible patients received up to four induction cycles of 177Lu-DOTATATE every eight weeks with activity tailored to achieve a cumulative renal absorbed dose of 26.5 Gy (assessed by quantitative SPECT/CT-based dosimetry). As clinical endpoints, the best radiological response (BRR), the PFS according to RECIST v1.1 criteria, the OS, and the safety were assessed. Subgroup analyses were conducted in Midgut NET patients meeting NETTER-1 eligibility criteria and in pNET patients [6].
A total of 226 patients were enrolled with the majority being males (58 %, n=130). Overall, 21 % of patients (n=48) had a NET Grad 1, 47 % (n=105) a NET Grad 2 and 8 % (n=17) a NET Grad 3 tumor. The median age was 63 years. The primary tumors of the patients were predominantly located in the midgut (40 %) and pancreas (37 %). The primary metastasis sites were the liver (88 %), lymph nodes (72 %) and bones (46 %). Prior systemic treatments included somatostatin analogues (88 %), everolimus (30 %), sunitinib (6 %), CAPTEM (6 %) or other chemotherapy regimens (15 %).
Overall, patients received 813 induction cycles containing an average of 9.54 GBq of 177Lu-DOTATATE per cycle. The cumulative renal dosimetry accounted for 22.91 Gy (2.08-35.66 Gy). The 4th induction cycle was completed by 74 % of patients with a cumulative renal dosimetry of 25.56 Gy.
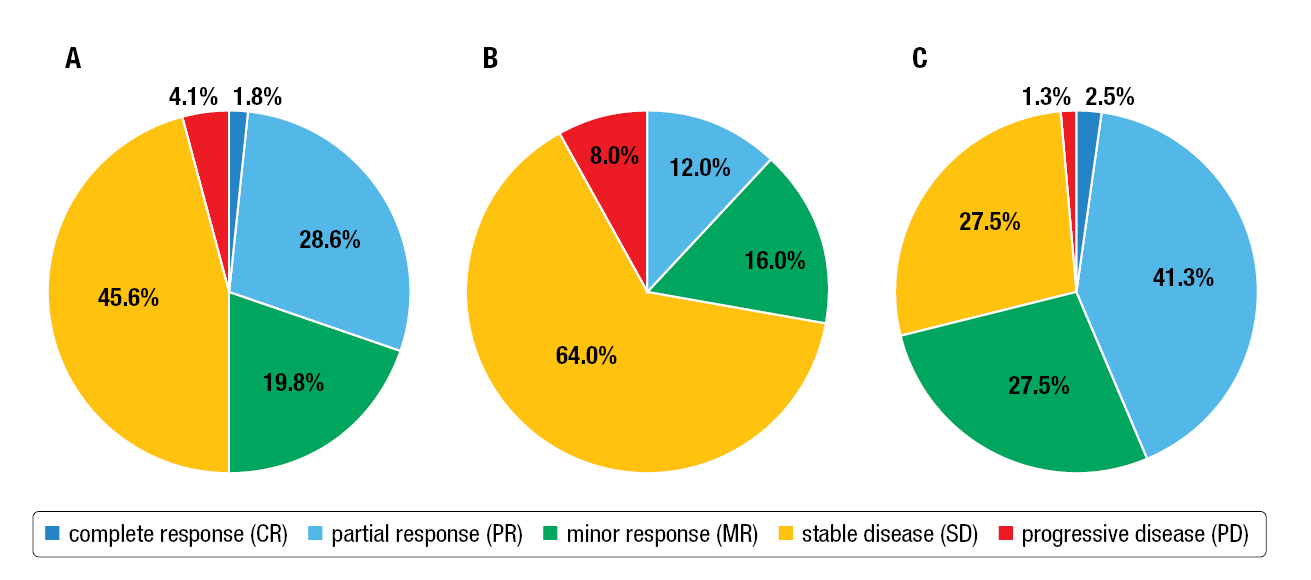
In the overall population (n=217), the ORR was 39.4 % with a DCR of 95.9 %. In the NETTER-1 subgroup (n=50), the ORR was 12.0 % with a DCR of 92.0 %, while in the pNET subgroup (n=80), the ORR was 43.8 %, with a DCR of 98.8 %, respectively (Figure 2). Median PFS reached 26.0 months in the overall population, 36.0 months in the NETTER-1 subgroup and 24.4 months in the pNET subgroup. The median OS was 44.0 months in the overall population, 42.3 months in the NETTER-1 subgroup and 44.0 months in the pNET subgroup.
The main subacute toxicities (< 12 months) experienced by the patients were thrombocytopenia (14.6 %), leucopenia (11.5 %), anemia (11.1 %), and neutropenia (8.8 %). The most frequent chronic toxicities (> 12 months) were thrombocytopenia (2.4 %), leucopoenia (3.2 %), anemia (3.2 %), and neutropenia (1.6 %) as well as renal impairment (2.4 %). Overall, 44 % of these AEs occurred after the fourth induction cycle, while in 9 %, the induction cycle was interrupted because of AEs. Of note, three patients (1.3 %) developed a myelodysplastic syndrome and one patient (0.4 %) an acute myeloid leukemia.
Based on these findings, the authors concluded that personalized 177Lu-DOTATATE PRRT, guided by renal dosimetry, demonstrated promising efficacy and a tolerable safety profile. These encouraging results may pave the way for higher injected activity in patients presenting with overexpressing SSTR NETs.
Figure 2: Best radiological response (BRR) of the overall population (A), the NETTER-1 (B) and the pNET subgroup (C).
Outcome prediction in GEP-NETs treated with 177Lu-DOTATATE PRRT
Although PRRT has shown efficacy in managing patients with advanced NETs that express SSTRs, there is a need for a strong imaging biomarker to predict PRRT efficacy [12, 13]. Magdalena Mileva presented the results from the LuMEn study (177Lu-octreotate treatment prediction using multimodality imaging in refractory NETs), a prospective, monocentric phase II clinical-imaging study in metastatic or locally advanced, non-resectable histologically proven GEP-NET patients (NCT01842165) [12]. The study aimed to determine if multimodality imaging parameters and the tumor absorbed dose are reliable early predictors for the outcome of patients with GEP-NETs during treatment with 177Lu-DOTATATE PRRT. Lesion-based time to progression (TTP) serves as primary endpoint, while PFS and best morphological response (according to RECIST v1.1) were secondarily analyzed.
Enrolled patients were treated with four cycles of 7.4 GBq of 177Lu-DOTATATE, given 11 to 13 weeks apart and injected intravenously with simultaneous infusion of an amino acid solution. At baseline and 10-12 weeks after the first injection, 68Ga-DOTATATE-PET/CT and FDG-PET/CT were performed. A maximum of five target lesions per patients were used to measure the specific uptake parameters (SUVmax/mean, tumor-to-blood, tumor-to-spleen ratio) and volumetric parameters (SSTR-tumor volume [TV], total-lesion SSTR expression).
The 37 patients included in the study had a mean age of 66 years and 51 % were men. The primary tumors were mainly located in the small-intestine (62 %), the pancreas (27 %) and the colon/rectum (11 %). Most of the tumors were classified as grade 1 (32 %) or grade 2 (59 %) and less frequently as grade 3 (8 %). The metastases were predominantly found in the liver (86 %), lymph nodes (84 %), bones (59 %) and peritoneum (32 %). In total, 75 % of patients received four PRRT-cycles.
Regarding the overall population, the median PFS was 28 months, and 30 % of patients achieved a PR. The median lesion TTP was not reached yet. After a median follow-up of 57 months, 14 % of target lesions have progressed. A patient-based analysis combining imaging parameters revealed that a decrease of SSTR-TV of ≥ 10 % after C1 from baseline was associated with a longer PFS of 51.3 months compared to 22.8 months for patients with
< 10 % decrease (p = 0.003; HR: 0.35; 95 % CI: 0.16-0.75). Similarly, an absorbed dose of ≥ 35 Gy received by all target lesions in C1 was associated with a longer PFS compared to an absorbed dose of < 35 Gy (48.1 vs 26.2 months; p = 0.02; HR: 0.37; 95 % CI: 0.17-0.82). Of note, neither the uptake parameters on 68Ga-DOTATATE PET/CT (baseline and changes after C1), nor the average and maximal tumor absorbed dose were associated with the PFS.
To conclude, in patients with GEP-NETs, changes in the volumetric parameter on 68Ga-DOTATATE-PET/CT after the first cycle of 177Lu-DOTATATE treatment might be useful for early response assessment. However, further studies are warranted.
REFERENCES
- Claringbold PG et al. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm 2012; 27(9): 561-569
- Kunz PL et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol 2023; 41(7): 1359-1369
- Claringbold PG et al. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology 2016; 103(5): 432-439
- Merola E et al. Peptide receptor radionuclide therapy (PRRT): innovations and improvements. Cancers (Basel) 2023; 15(11): 2975
- Cwikla JB et al. Evaluation of progression-free survival (PFS) in patients with advanced, non-resectable, progressive GEP-NET treated using combine radioligand and CAPTEM therapy.
EANM 2023 (Oral abstract OP-231) - Strosberg J et al. Phase 3 Trial of (177)Lu-DOTATATE for midgut neuroendocrine tumors.
N Engl J Med 2017; 376(2): 125-135 - Mitjavila M et al. Efficacy of [(177)Lu]Lu-DOTATATE in metastatic neuroendocrine neoplasms of different locations: data from the SEPTRALU study. Eur J Nucl Med Mol Imaging 2023; 50(8): 2486-2500
- Mitjavila M et al. Efficacy and safety of 177Lu-DOTATATE in lung neuroendocrine tumors: a multicenter study. EANM 2023 (Oral abstract OP-233)
- Naraev BG et al. Peptide receptor radionuclide therapy for patients with advanced lung carcinoids. Clin Lung Cancer 2019; 20(3): e376-e392
- Del Prete M et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging 2019; 46(3): 728-742
- Morin MA et al. Efficacy and safety of dosimetry-based, personalized 177Lu-DOTATATE PRRT of neuroendocrine tumours: an update from the P-PRRT trial. EANM 2023 (Oral abstract OP-236)
- Mileva M et al. Outcome prediction in patients with gastroenteropancreatic neuroendocrine tumours (GEPNETs) treated with 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT): results from a prospective phase II clinical trial. EANM 2023 (Oral abstract OP-238)
- Liberini V et al. The challenge of evaluating response to peptide receptor radionuclide therapy in gastroenteropancreatic neuroendocrine tumors: The present and the future. Diagnostics (Basel) 2020; 10(12):1083
© 2023 Springer-Verlag GmbH, Impressum
More posts
Theranostics: recent developments in neuroendocrine tumors
Theranostics: recent developments in neuroendocrine tumors 225Ac-DOTATATE daughter nuc