No phase III benefit with selumetinib in KRAS-mutant NSCLC
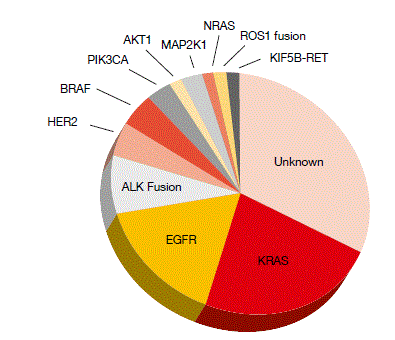
Oncogenic mutations of KRAS define the largest genomic subset of NSCLC (Figure). This patient group appears to derive less clinical benefit from chemotherapy than the overall NSCLC population. There are currently no targeted treatments specifically for patients with KRAS-mutant tumours of the lung. However, KRAS mutations are associated with activation of the RAS/RAF/MEK/ERK pathway, which converges at MEK1/2, making KRAS mutation in NSCLC a potential target of the oral MEK1/2 inhibitor selumetinib. Indeed, in a phase II trial, selumetinib has shown encouraging activity in combination with docetaxel, improving PFS and ORR to a significant extent compared to placebo plus docetaxel [1].
The phase III SELECT-1 study therefore tested selumetinib 75 mg twice daily plus docetaxel against placebo plus docetaxel in patients with KRASmutated advanced NSCLC (stage IIIB-IV) after failure of first-line therapy [2]. PFS by investigator assessment was defined as the primary endpoint. Overall, 510 patients were randomised. SELECT-1 was the first and largest prospective phase III, randomised, double-blind trial of second-line treatment for patients with KRAS-mutant NSCLC.
Figure: Molecular subsets of adenocarcinoma of the lung
However, PFS did not differ significantly between the treatment arms (3.9 vs. 2.8 months with selumetinib plus docetaxel and docetaxel alone, respectively; HR, 0.93). This also applied to OS (8.7 and 7.9 months, respectively; HR, 1.05). There was no evidence of a statistically significant interaction of treatment by subgroup with regard to both PFS and OS. ORR was numerically improved in the experimental arm (confirmed responses, 13 % vs. 9 %); however, responses lasted only 2.9 months (vs. 4.5 months in the control arm). The safety profile of selumentinib plus docetaxel was consistent with historical data for docetaxel and emerging data for selumetinib.
At present, there is no clear reason why the phase II results did not translate into a positive phase III study. Exploratory analyses are ongoing or planned for different KRAS codon mutations, as well as for PD-L1, LKB1 and TP53 status.
REFERENCES
- Jänne PA et al., Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebocontrolled, phase 2 study. Lancet Oncol 2013; 14: 38-47
- Jänne PA et al., Selumetinib in combination with docetaxel as second-line treatment for patients with KRAS-mutant advanced NSCLC: results from the phase III SELECT-1 trial. ESMO 2016, abstract LBA47_PR