Update on the treatment for advanced HCC
With more than 900,000 newly diagnosed cases in 2020, hepatocellular carcinoma (HCC) is the sixth most frequent malignant disease with a high mortality rate of approximately 92 % [1]. Current first-line treatment for advanced HCC includes atezolizumab plus bevacizumab [2], as well as the tyrosine kinase inhibitors (TKIs) sorafenib [3, 4] and lenvatinib [5].
Numerous trials are ongoing to investigate first- or second-line mono- or combination therapies to further improve response and survival rates in patients with advanced HCC.
1L camrelizumab plus rivoceranib in unresectable HCC
In advanced HCC, immune checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1 did not provide any survival benefit over sorafenib in the first-line setting when used as single immunotherapeutic agents so far [6]. However, adding an anti-angiogenic tyrosine kinase inhibitor (TKI) to ICI monotherapy has shown promising improvements of survival outcomes in renal cell carcinoma and endometrial carcinoma [7]. Camrelizumab, an anti-PD-1 IgG4 antibody, and rivoceranib (also known as apatinib), a small-molecule VEGFR2-targeted TKI, have both been approved in China as second-line monotherapy agents in advanced HCC [8, 9]. Interestingly, camrelizumab in combination with rivoceranib showed encouraging preliminary antitumor activity and an acceptable tolerability in pretreated HCC in a phase I trial [10]. Moreover, a phase II study in patients with advanced HCC demonstrated promising efficacy with an overall response rate (ORR) of 34.3 %, a median progression-free survival (mPFS) of 5.7 months and an 18-month overall survival (OS) rate of 58.1 % [11]. A phase III trial (NCT03764293) presented at ESMO 2022 compared the efficacy and safety of this dual first-line therapy versus sorafenib in unresectable HCC [12].
Patients matching key eligibility criteria (including unresectable or metastatic HCC, BCLC stage B or C, no prior systemic therapy, ECOG 0 or 1, Child-Pugh score A and more than 1 measurable lesion as assessed by RECIST v1.1) were randomized 1:1 to receive either camrelizumab (200 mg, intravenously [IV], every second week [Q2W]) plus rivoceranib (250 mg, per os [PO], twice daily [BID]) or sorafenib (400 mg, PO, BID). The co-primary endpoints included PFS and OS. ORR was set as a key secondary outcome. PFS and ORR were assessed by a blinded independent central review (BICR) per RECIST v1.1. Patients were stratified by tumor extent (extra-hepatic spread/macrovascular invasion or not), geographic origin (Asian or not) and baseline serum alpha-fetoprotein (< or ≥ 400 ng/mL).
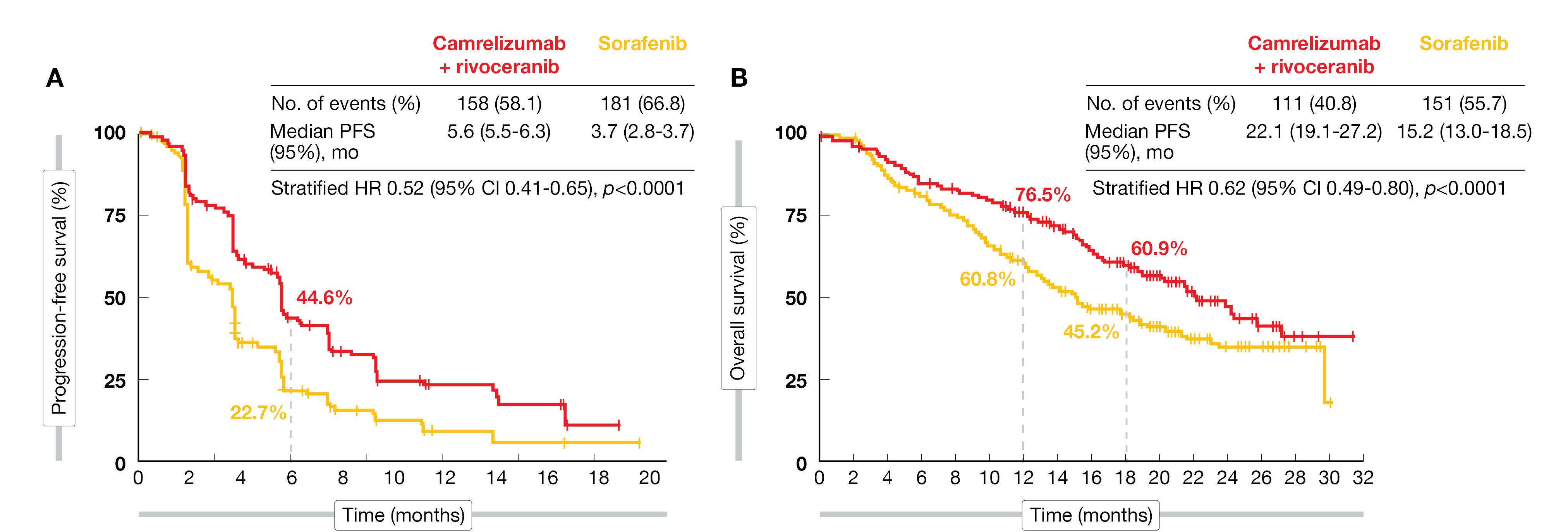
Out of the 543 enrolled patients, 272 were included in the camrelizumab plus rivoceranib ITT population versus 271 in the sorafenib ITT population. At the time of data cut-off (February 8, 2022), 42 and 22 patients, respectively, were still receiving their treatment. A significant benefit of camrelizumab plus rivoceranib compared to sorafenib was demonstrated for both PFS (median PFS: 5.6 vs 3.7 months; HR, 0.52; 95 % CI, 0.41-0.65; one-sided p<0.0001) (Figure 1A) and OS (median OS: 22.1 vs 15.2 months; HR, 0.62; 95 % CI, 0.49-0.80; one-sided p<0.0001) (Figure 1B). A pre-specified subgroup analysis showed that HRs of PFS and OS obviously favored camrelizumab plus rivoceranib in most subgroups. The ORR was also significantly improved in the camrelizumab plus rivoceranib arm versus sorafenib (25.4 % [1.1 % complete response/CR, 24.3 % partial responses/PR] vs 5.9 % [0.4 % CR, 5.5 % PR]; p<0.0001), while the disease control rate (DCR) was 78.3 % and 53.9 %, respectively. The median duration of response (DoR) reached 14.8 months in the camrelizumab plus rivoceranib arm versus 9.2 months in the sorafenib arm. Moreover, the rate of patients with reduction in the sum of diameters of target lesions was twice as high in the combination arm compared to sorafenib (72.8 % vs 35.6 %).
Grade ≥ 3 treatment-related adverse events (TRAEs) were more frequent within the combination arm (80.5 %), with hypertension (37.5 %), increased aspartate aminotransferase (16.5 %) and alanine aminotransferase (12.9 %) being the most frequent ones; compared to sorafenib (52.0 %), patients experienced most commonly palmar-plantar erythrodysaesthesia (15.2 %), hypertension (14.9 %) and increased gamma-glutamyl transferase (7.4 %). One patient in each treatment arm experienced a grade 5 TRAE. The number of TRAEs leading to discontinuation of treatment was similar in both groups (10 vs 12, respectively).
This first positive international phase III study of a TKI combined with an anti-PD1 agent supports this combination of rivoceranib plus camrelizumab as another new first-line treatment option for unresectable HCC.
Figure 1: Progression-free survival (A) and overall survival (B) in the ITT population.
RATIONALE-301: 1L tislelizumab monotherapy
Several single-agent ICI have been or are currently under evaluation in HCC [6, 13, 14]. Among those, tislelizumab – a new monoclonal antibody with high binding affinity for PD-1 – is currently being investigated as first-line treatment in adult patients with unresectable HCC. This antibody has been specifically engineered to minimize Fcγ receptor binding on macrophages because this was shown to have a negative impact on the anti-PD-1 antibody-mediated antitumoral activity [15, 16]. In the phase II RATIONALE-208 study (NCT03419897) durable responses and generally good tolerability of tislelizumab monotherapy were shown in patients with previously treated advanced HCC [17]. At this year’s ESMO meeting, Kudo et al. presented the final analysis of the RATIONALE-301 trial, which evaluated the safety and the efficacy of first-line tislelizumab monotherapy versus sorafenib in patients with unresectable HCC [14].
RATIONALE-301 is a randomized, open-label, multicenter phase III study (NCT03412773). Eligible patients had histologically confirmed HCC, no prior treatment, BCLC stage C or B disease with no progression risk or event after loco-regional therapy, Child-Pugh score A, ≥ 1 lesions assessed by RECIST v1.1, no tumor thrombus, and ECOG 0 or 1. Patients were randomized 1:1 to receive either tislelizumab (200 mg, IV, Q3W) or sorafenib (400 mg, PO, BID) until disease progression or intolerable toxicity. The primary endpoint was OS in the ITT population, while other efficacy parameters (ORR, PFS and DoR) assessed by BIRC according to RECIST v1.1 and safety were secondarily analyzed. Stratifications factors included macrovascular invasion, extrahepatic spread, ECOG performance status, etiology, and geography.
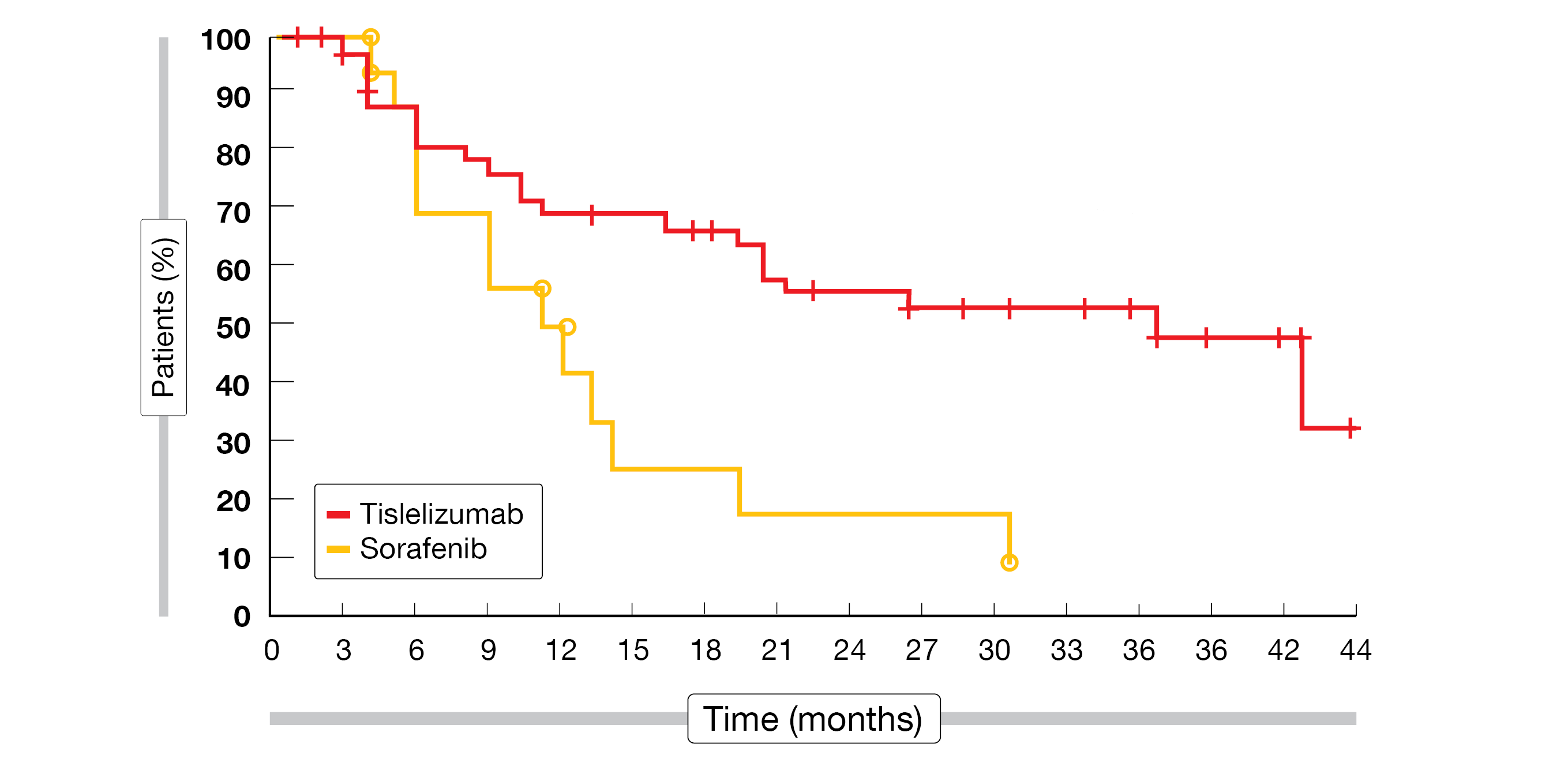
Patients in the tislelizumab arm (n = 342) and the sorafenib group (n = 332), who were followed for a minimum of 33 months, had a median age of 62 and 60 years, respectively. According to the final median OS analysis, tislelizumab monotherapy demonstrated non-inferiority versus sorafenib (15.9 vs 14.1 months; stratified HR, 0.85; 95 % CI, 0.712-1.019; p = 0.0398). These results were consistently observed across all prespecified subgroups. There was a benefit of tislelizumab (CR, 2.9 %; PR, 11.4 %) in terms of ORR (14.3 % vs 5.4 %) compared to sorafenib (CR, 0.3 %; PR, 5.1 %) and in terms of median DoR (36.1 vs 11.0 months) (Figure 2). Of note, the median PFS was shorter with tislelizumab than with sorafenib (2.1 vs 3.4 months; stratified HR, 1.11; 95 % CI, 0.92-1.33).
Tislelizumab was well tolerated as it was associated with less grade ≥3 treatment-emergent AEs (TEAEs) (48.2 % vs 65.4 %) and TRAEs (22.2 % vs 53.4 %) compared to sorafenib. The most frequent TEAEs associated with tislelizumab were increased aspartate aminotransferase (AST), alanine amino-
transferase (ALT), and blood bilirubin, while those associated with sorafenib were PPES, diarrhea, and increased AST. Treatment discontinuations (10.9 % vs 18.5 %) and dose modifications (31.1 % vs 64.8 %) following TEAEs were less frequent in the tislelizumab arm compared to sorafenib.
RATIONALE-301 met its primary endpoint of OS non-inferiority with tislelizumab versus sorafenib demonstrating a clinically meaningful antitumor benefit with a favorable and manageable safety profile as 1L monotherapy for patients with unresectable HCC.
Figure 2: Duration of response in the ITT population of the RATIONALE-301 study.
LEAP-002: 1L lenvatinib plus pembrolizumab
In the KEYNOTE-224 phase II trial, pembrolizumab has been evaluated as second-line monotherapy in advanced HCC; these results led to pembrolizumab’s approval in the US after demonstrating promising ORR in sorafenib-pretreated patients [18]. Two phase III studies in HCC demonstrated a favorable benefit/risk profile despite a narrowly missed statistical significance for OS and PFS (KEYNOTE-240 global study [19]), significantly improved OS, PFS and ORR (KEYNOTE-394 Asian study [20]) and consistent improvements in OS, PFS and ORR (KEYNOTE-240 and KEYNOTE-394 meta-analysis [21]). Lenvatinib – a TKI inhibitor – has been approved as 1L treatment of advanced HCC after demonstrating OS non-inferiority versus sorafenib (REFLECT study [5]). The combination of lenvatinib plus pembrolizumab showed promising efficacy and safety in 1L unresectable HCC in a phase Ib study (study 111/KEYNOTE-524) [22]. At ESMO 2022, the outcomes of the LEAP-002 study investigating the first-line combination pembrolizumab plus lenvatinib in advanced HCC were presented [23].
The LEAP-002 study is a global, double-blind, placebo-controlled phase III study assessing lenvatinib plus pembrolizumab versus lenvatinib monotherapy in 1L advanced HCC (NCT03713593). Enrolled patients had confirmed HCC, were not eligibility to curative therapy, had no prior treatment, ECOG 0 or 1, Child-Pugh score class A, an esophagogastroduodenoscopy (EGD) within the last three months and no major portal invasion. Randomization occurred 1:1 with patients being administered lenvatinib (8 mg if body weight [BW] < 60 kg or 12 mg if BW > 60 kg, PO, BD) either combined with pembrolizumab (200 mg, IV, Q3W) or plus placebo (saline, IV, Q3W) for a maximum of 35 cycles. Dual primary endpoints were OS and PFS assessed by BICR according to RECIST v1.1. Secondary endpoints included ORR and DoR, assessed by BICR per RECIST v1.1 or mRECIST, as well as safety/tolerability. Patients were stratified by geographic region, macroscopic portal vein invasion/extrahepatic spread, alpha-fetoprotein (AFP) level and ECOG performance status.
A total of 794 patients were randomized from January 17, 2019, to April 28, 2020, resulting in 395 patients in the lenvatinib plus pembrolizumab arm and 399 patients in the lenvatinib plus placebo arm. Median age was 66 years in both groups. Median follow-up was 17.6 months for final PFS and 32.1 months for the final OS analysis. LEAP-002 did not meet its pre-specified statistical significance for both primary endpoints as neither the median OS (21.2 vs 19.0 months; HR, 0.840; 95 % CI, 0.708-0.997; p = 0.0227), nor the median PFS (8.2 vs 8.0 months; HR, 0.867; 95 % CI, 0.734-1.024; p = 0.0466) reached prespecified superiority thresholds in the final analysis for lenvatinib plus pembrolizumab versus lenvatinib plus placebo. However, a subgroup OS analysis favored lenvatinib plus pembrolizumab versus lenvatinib plus placebo in patients ≥ 65 years, macroscopic portal vein invasion/extrahepatic spread, hepatitis B virus or AFP levels > 400 ng/mL. More patients in the lenvatinib plus pembrolizumab arm attained an objective response (ORR, 26.1 % vs 17.5 %) with a longer DOR (16.6 vs 10.4 months).
No new safety concerns were reported. The frequency of TRAEs events was similar in both arms, with a higher number of grade 3-4 TRAEs in the lenvatinib plus pembrolizumab group (61.5 % vs 56.7 %), with hypertension, proteinuria, decreased platelet count, increased AST levels and diarrhea being the most frequently reported TRAEs. The number of treatment discontinuation was higher in the combination arm (18.0 % vs 10.6 %). Immune-mediated AEs (imAEs) and infusion reactions grade 3-4 were more frequent in the group receiving lenvatinib plus pembrolizumab than in the lenvatinib plus placebo group (8.9 % vs 2.3 %) – with 9.6 % and 1.8 % of patients requiring systemic corticoids.
As the study did not meet its pre-specified statistical significance for the primary endpoints of OS and PFS, this combination will not be added to the treatment algorithm of advanced stage HCC. A phase III study (LEAP-012) testing the combination of transarterial chemoembolization (TACE) plus lenvatinib and pembrolizumab is currently under investigation (NCT04246177).
KEYNOTE-240: 2L pembrolizumab monotherapy
In sorafenib or oxaliplatin-based chemotherapy-treated advanced HCC patients from Asia, pembrolizumab demonstrated statistically significant and clinically meaningful benefit in the KEYNOTE-394 trial [20]. Although in the KEYNOTE-240 phase III trial, the statistical significance criteria for OS and PFS were narrowly missed [19], ORR data were consistent with those of the KEYNOTE-224 study [18], which led to the approval of pembrolizumab in the United States. At ESMO 2022 the updated outcomes from the KEYNOTE-240 trial after a 4.5-year follow-up were presented [24].
KEYNOTE-240 is a randomized, double-blind phase III study comparing pembrolizumab monotherapy with placebo in a second-line setting in participants with sorafenib-pretreated advanced HCC (NCT02702401). Patients with histologically, cytologically, or radiographically confirmed advanced HCC were randomly assigned at a 2:1 ratio to receive either pembrolizumab (200 mg, IV, Q3W) plus best supportive care (BSC) or placebo plus BSC for up to 35 cycles. Key eligibility criteria included a measurable disease per RECIST v1.1, a progression or intolerance to sorafenib, Child-Pugh liver class A, BCLC stage C or B not amenable or refractory to locoregional therapy and not amenable to curative treatment and ECOG 0 or 1. Dual primary endpoints were OS and PFS assessed by RECIST v1.1 by BICR, while ORR, DoR, DCR, time to progression (TTP) and safety were the secondary endpoints.
At the time of this updated analysis (data cutoff: September 22, 2021), the median follow-up time was 53.9 months for pembrolizumab (n = 278) and 54.1 months for placebo (n = 135). Compared to placebo, pembrolizumab treatment showed an improved median OS (13.9 vs 10.6 months; HR, 0.77; 95 % CI, 0.62-0.96), as well as median PFS (3.0 vs 2.8 months; HR, 0.73; 95 % CI, 0.58-0.91). Concerning the secondary endpoints, the ORR was higher in the pembrolizumab arm (18.3 %, including 10 patients with a CR and 41 with a PR) than in the placebo arm (4.4 %, including no patients with a CR and 6 patients with a PR). Overall, 63.3 % of pembrolizumab-treated patients versus 55.6 % of placebo patients were able to reach a disease control (DCR). The median DOR reached 13.9 months in the pembrolizumab arm versus 15.2 months in the placebo arm, while the TTP was 3.8 versus 2.8 months, respectively.
TRAEs grade 3-4 accounted for 19.7 % in the pembrolizumab arm and 7.5 % in the placebo arm. The most frequent grade 3 or 4 TRAEs were increased AST (5.7 % with pembrolizumab vs 1.5 % with placebo) and increased ALT (3.9 % vs 1.5 %). The immune-mediated AEs and infusion-related reactions were mild in severity (grade 3-4, 7.2 % in pembrolizumab arm vs 0.7 % in placebo arm).
The updated results of the KEYNOTE-240 study, together with data from KEYNOTE-224 and KEYNOTE-394, provide further evidence for a favorable benefit-risk profile of pembrolizumab monotherapy as treatment for patients with advanced HCC.
REFERENCES
- Sung, H, et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Finn, RS, et al., Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020; 382(20): 1894-1905.
- Cheng, AL, et al., Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25-34.
- Llovet, JM, et al., Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359(4): 378-390.
- Kudo, M, et al., Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391(10126): 1163-1173.
- Yau, T, et al., Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022; 23(1): 77-90.
- Huinen, ZR, et al., Anti-angiogenic agents – overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol 2021; 18(8): 527-540.
- Qin, S, et al., Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2021; 6(7): 559-568.
- Qin, S, et al., Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020; 21(4): 571-580.
- Xu, J, et al., Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res 2021; 27(4): 1003-1011.
- Xu, J, et al., Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res 2019; 25(2): 515-523.
- Qin, S, et al., Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 2022; 33(suppl 7; Abstr LBA35).
- Abou-Alfa, GK, Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol 2022; 4: 379-379.
- Qin, S, et al., Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022; 33(suppl 7; Abstr LBA36).
- Zhang, T, et al., The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Hong, Y, et al., Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio 2021; 11(3): 782-792.
- Ducreux, M, Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with previously treated advanced hepatocellular carcinoma. Ann Oncol 2021; 32(suppl 3; Abstr 0-1).
- Zhu, AX, et al., Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19(7): 940-952.
- Finn, RS, et al., Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020; 38(3): 193-202.
- Qin, SK, Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol 2022; 40(Suppl 4; Abstr 383).
- Finn, RS, Pembrolizumab (pembro) for previously treated advanced hepatocellular carcinoma (aHCC): Meta-analysis of the phase 3 KEYNOTE-240 and KEYNOTE-394 studies. Cancer Research 2022; 82(Suppl 12): CT222.
- Finn, RS, et al., Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020; 38(26): 2960-2970.
- Finn, RS, et al., Primary results from the phase III LEAP-002 study:Lenvatinib plus pembrolizumab versus lenvatinib as first-line(1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022; 33(suppl 7; Abstr LBA34).
- Edeline, J, Pembrolizumab vs placebo as second-line treatment for sorafenib-treated advanced hepatocellular carcinoma: 4.5-year follow-up from KEYNOTE-240. Ann Oncol 2022; 33(suppl 7; Abstr 713P).
© 2022 Springer-Verlag GmbH, Impressum
More posts
New clinical insights in head and neck squamous cell carcinoma
New clinical insights in head and neck squamous cell carcinoma Head and neck squamous c
Preface – ESMO Solid Tumor 2022
Preface – ESMO Solid Tumors 2022 © privat - Jaime R. Merchan, MD. MMSc, University of