Immunotherapy: the emerging paradigm of cure
The introduction of molecularly targeted agents 15 to 20 years ago marked the beginning of a new era. Today, immune-checkpoint–inhibiting drugs have established yet another level of treatment. While both chemotherapy and targeted agents exert their effects directly at the tumor, which implies the eventual emergence of resistance, immunotherapy targets the immune system, enabling a certain percentage of patients to survive over extended periods of time. Patients with advanced non–small-cell lung cancer (NSCLC) who are alive at 2 years after treatment initiation have a realistic chance to live on for more than 5 years. Thus, cure in advanced cancer has emerged as a new paradigm.
Three types of immune checkpoint inhibitors are currently in use: anti-CTLA-4 antibodies (e.g., ipilimumab, tremelimumab), anti-PD-1 antibodies (e.g., nivolumab, pembrolizumab), and anti-PD-L1 antibodies (e.g., atezolizumab, durvalumab, avelumab).
Advantages of first-line treatment
The anti-PD-1 antibody pembrolizumab outperformed chemotherapy with docetaxel in the KEYNOTE-010 study even though patients were pretreated [1]. The group with at least 50 % of tumor cells expressing PD-L1 (tumor proportion score [TPS] ≥ 50 %) derived the greatest benefit. Here, pembrolizumab treatment led to significant improvements in both overall survival (OS) and progression-free survival (PFS).
In the first-line setting, the KEYNOTE-024 trial evaluated pembrolizumab 200 mg every 3 weeks over 2 years compared to platinum-doublet chemotherapy for 4-6 cycles in 305 patients with a PD-L1 TPS of ≥ 50 % [2]. Crossover from the control arm to the experimental arm was permitted in case of disease progression; at that time, patients went on to receive the pembrolizumab regimen administered in the experimental arm.
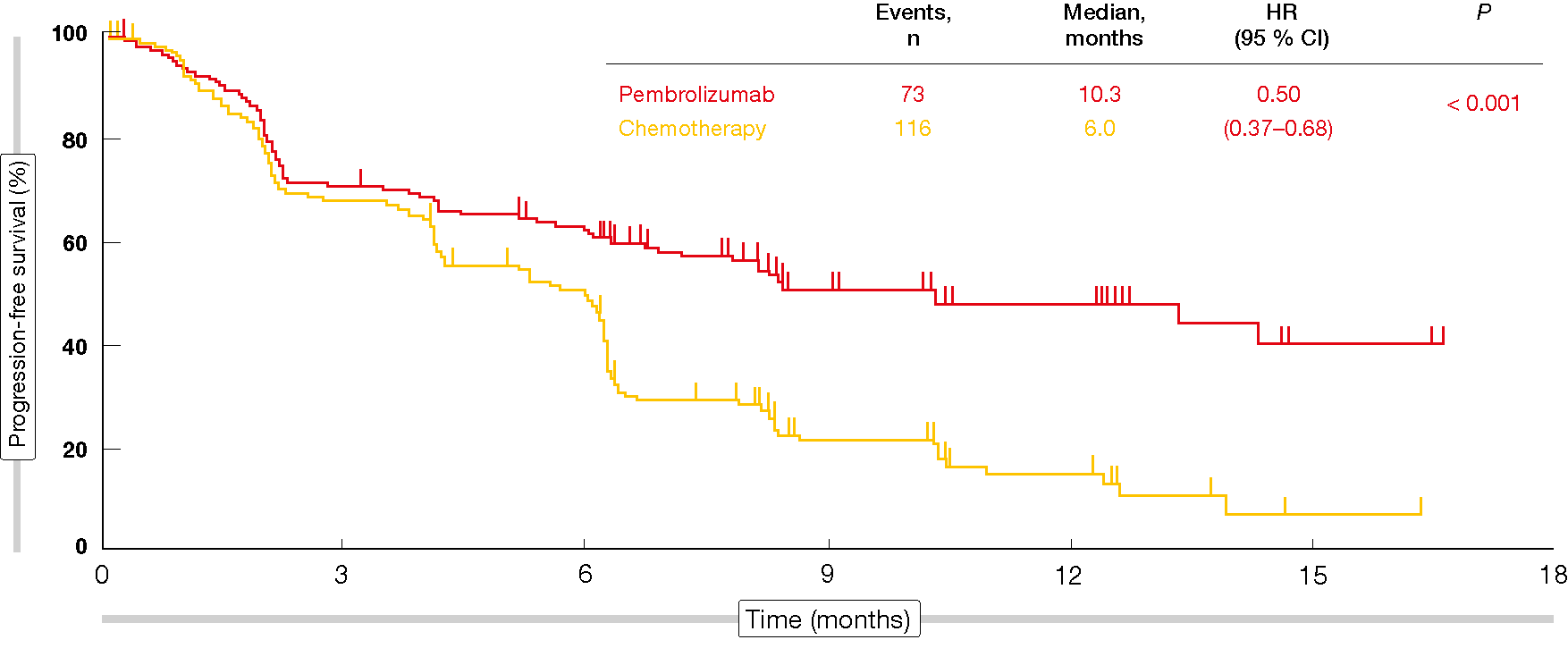
The analysis revealed a significant PFS difference in favor of pembrolizumab (10.3 vs. 6.0 months; HR, 0.50; p < 0.001; Figure 1). In spite of the crossover, patients in the experimental arm fared significantly better with respect to OS than those in the control arm (not reached in either group; HR, 0.60; p = 0.005). According to the updated results of KEYNOTE-024, a high degree of separation of the OS curves was maintained despite an effective crossover rate of 60 % [3]. All of this suggests that immunotherapy should be administered from the beginning rather than after chemotherapy.
Figure 1: Progression-free survival obtained with pembrolizumab vs. chemotherapy in KEYNOTE-024
Biomarkers other than PD-L1 expression
Gettinger et al. described the characteristics of 16 patients surviving for 5 years in the phase I CA209-003 study investigating nivolumab in the pretreated setting [4]. Nivolumab had been discontinued after 2 years of treatment. Apparently, initial responses predicted long-term survival, as most of the patients (75 %) had achieved partial remission (PR) soon after the start of the study.
However, absence of response constitutes a major issue in the context of immunotherapy. Approximately one third of patients do not respond to treatment, even in the presence of high PD-L1 expression. Tumors grow rapidly in the majority of cases, and prognosis is poor. PD-L1 expression does not suffice as a biomarker here, as it results from the interaction between tumor cells and the immune system and therefore lacks stability.
A Japanese study identified the expression of the homing molecule CD62L on T cells as a potential biomarker [5]. The researchers evaluated this option based on the hypothesis that distinct pre-existing anti-tumor immunity in certain patients might lead to different responses to anti-PD-1 therapy. In a group of 50 consecutive NSCLC patients who were treated with nivolumab, those achieving PR or stable disease (SD) were shown to have significantly more CD4-positive T cells down-regulating CD62L (i.e., CD62Llow) than those experiencing progressive disease (p = 4.1 x 10-7). The percentages of CD62Llow in CD4-positive T cells provided sensitivity of 92.9 % and specificity of 96.7 % with regard to the prediction of progression. Moreover, SD patients had a significantly smaller regulatory T cell subpopulation than the PR population (p = 0.0067), which means that it was possible to predict PR from SD. A prospective study investigating these findings is already ongoing in Japan.
Combination therapy
The KEYNOTE-189 trial assessed first-line pembrolizumab in combination with pemetrexed and cisplatin or carboplatin for 4 cycles in non-squamous NSCLC [6]. Patients in the control arm received placebo instead of pembrolizumab together with the other components as described for the experimental arm. The protocol stipulated no enrichment by PD-L1 expression status, although this was a stratification factor.
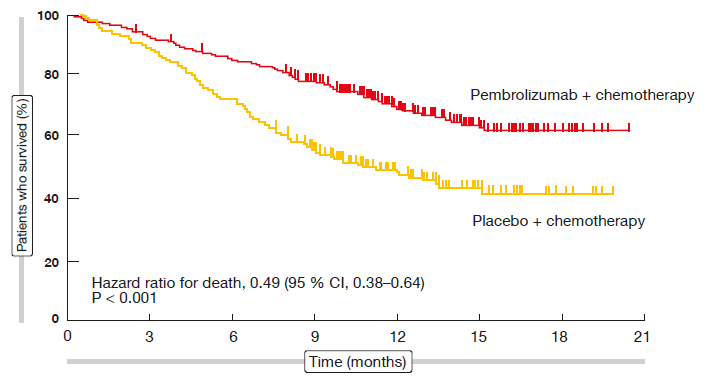
The addition of pembrolizumab to standard chemotherapy resulted in significant improvements in PFS (HR, 0.52; p < 0.001) and OS (HR, 0.49; p < 0.001; Figure 2) compared to chemotherapy alone. At 12 months, 69.2 % vs. 49.4 % of patients were alive. The separation of both PFS and OS curves commenced directly after treatment initiation. Both PFS and OS benefits were most pronounced in the group with PD-L1 expression ≥ 50 %, even though patients showing lower expression levels also fared better with the pembrolizumab-based regimen.
The ongoing KEYNOTE-407 study follows in the steps of KEYNOTE-189, investigating first-line chemotherapy with or without pembrolizumab in patients with tumors of squamous histology. At the ASCO 2018 Congress, the second interim analysis was presented [7]. As for KEYNOTE-189, the pembrolizumab combination was superior to chemotherapy alone regarding both PFS (6.4 vs. 4.8 months; HR, 0.56; p < 0.0001) and OS (15.9 vs. 11.3 months; HR, 0.64; p = 0.0008). Here, too, the results obtained in the experimental arm exceeded those in the control arm regardless of PD-L1 expression.
Overall, combinations of immunotherapy and chemotherapy appear to give rise to improved clinical outcomes irrespective of histology and PD-L1 expression status. These findings compare favorably to those obtained with immune checkpoint inhibitor monotherapy, which implies that most of the patients with advanced NSCLC will be treated with combination regimens once these have received approval. However, it must be kept in mind that this entails enormous healthcare costs. Monotherapy might be sufficient for some patients with high PD-L1 expression, although of course physicians would generally prefer to be on the safe side for the sake of the patient.
Figure 2: KEYNOTE-189: overall survival benefit in patients treated with pembrolizumab plus standard chemotherapy
Locally advanced NSCLC & neoadjuvant setting
Immunotherapy has not only excelled in metastatic disease, but also in the locally advanced setting. Patients with stage III, locally advanced, unresectable NSCLC that had not progressed following platinum-based chemoradiotherapy were treated with either durvalumab or placebo in the PACIFIC study [8]. Indeed, compared to placebo, durvalumab treatment gave rise to significant improvements in PFS (16.8 vs. 5.6 months; HR, 0.52; p < 0.001) and time to distant metastasis or death (23.2 vs. 14.6 months; HR, 0.52; p < 0.001).
Neoadjuvant treatment represents one step further to the very front lines. Here, a pilot study evaluated two preoperative doses of nivolumab in adults with untreated, surgically resectable early (stage I, II, or IIIA) NSCLC [9]. Surgery was performed approximately 4 weeks after the first dose. Neoadjuvant administration of nivolumab appeared feasible, with an acceptable side-effect profile. It did not delay surgery and induced major pathological responses in 9 of 20 resected tumors (45 %). Responses occurred in both PD-L1–positive and PD-L1–negative tumors.
Adverse effects with predicting capacity
Immune-related adverse events (irAEs) are not necessarily bad news. Beyond embodying a simple side effect, they can be a sign of reactivation of the patient immune response. Haratani et al. demonstrated that patients with irAEs, as compared to those without, had improved outcomes with regard to PFS (9.2 vs. 4.8 months; p = 0.04) and OS (not reached vs. 11.1 months; p = 0.01) [10]. Of course, irAEs can be life threatening and require appropriate management. Detailed guidelines have been established for this purpose [11].
REFERENCES
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540-50
- Reck M et al., Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375(19): 1823-1833
- Brahmer JR et al., Progression after the next line of therapy (PFS2) and updated OS among patients with advanced NSCLC and PD-L1 TPS >=50% enrolled in KEYNOTE-024. J Clin Oncol 35, 2017 (suppl; abstr 9000)
- Gettinger S et al., Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 Study. J Clin Oncol 2018; 36(17): 1675-1684
- Kagamu H et al., CD4+ T cells in PBMC to predict the outcome of anti-PD-1 therapy. J Clin Oncol 35, 2017 (suppl; abstr 11525)
- Gandhi L et al., Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078-2092
- Paz-Ares LG et al., Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (chemo) with or without pembrolizumab (pembro) for patients (pts) with metastatic squamous (sq) non-small cell lung cancer (NSCLC). J Clin Oncol 36, 2018 (suppl; abstr 105)
- Antonio SJ et al., Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017; 377: 1919-1929
- Forde PM et al., Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976-1986
- Haratani K et al., Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4(3): 374-378
- Haanen JBAG et al., Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (Supplement 4): iv119-iv142