CLL treatment in the real world: insights from across the globe
Second- and later-line therapies in the USA
The analysis reported by Davids et al. at iwCLL 2023 examined the characteristics, treatment patterns and outcomes of a cohort of 1,102 real-world US patients with CLL receiving two or more lines of therapy [1]. Data were obtained from the COTA real-world database. Second-line treatment was initiated between 2014 and 2021. The median patient age at diagnosis was 64 years, 61 % were males, and 88.1 % were treated in the community setting.
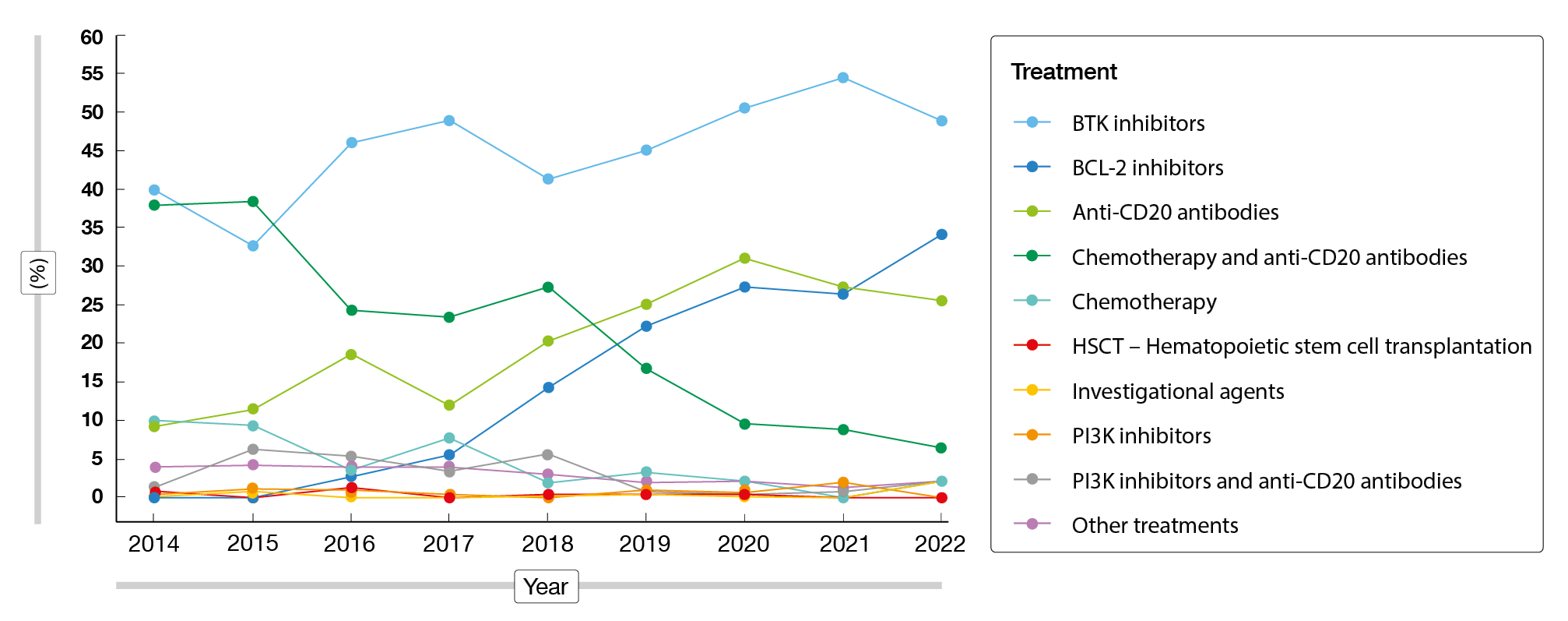
Second-line regimens predominantly included ibrutinib, bendamustine plus rituximab (BR), rituximab monotherapy, acalabrutinib, and investigational regimens. In the third line, patients most commonly received ibrutinib, acalabrutinib, and BR. Fourth-line regimens were mainly ibrutinib, rituximab plus venetoclax, and acalabrutinib. Between 2014 to 2022, the utilization of BTK inhibitors had increased from 39.9 % to 48.9 %; for BCL-2 inhibitors and anti-CD20 antibodies, these proportions had risen from 0.0 % to 34.0 % and from 9.2 % to 25.5 %, respectively (Figure 1). At the same time, the utilization of chemoimmunotherapy had decreased considerably from 37.9 % to 6.4 %.
Although targeted agents have improved patient outcomes, the findings suggested that there is still an unmet need. Second-line therapies were discontinued in 77.5 %. Toxicity was the reason for this in 29.0 %, physician preference in 9.4 %, progression in 6.4 % and death in 6.8 %. In almost half of cases, the reasons for discontinuation were classified as “other”. Approximately 18 % of patients died after the initiation of second-line therapies and prior to the start of the third line; 25.5 % of all patients died prior to the initiation of the fourth line. From the start of second-line treatment, median real-world progression-free survival (PFS) was 31.4 months, and median real-world overall survival (OS) was 79.0 months. The authors noted that innovative treatment options and novel mechanisms of action are required to improve outcomes in patients with CLL.
Figure 1: CLL treatment regimens administered in the USA between 2014 and 2022
Observations from China
In light of the scarcity of empirical evidence on the usage of BTK inhibitors in China, a retrospective cohort study including 673 patients with B-cell lymphoproliferative diseases was performed to assess real-world treatment patterns, discontinuation rates and clinical outcomes on BTK inhibitor therapy [2]. Median duration of treatment was 36.4 months in the entire cohort, and median post–BTK-inhibitor survival had not been reached yet. During follow-up, 43.8 % of patients permanently discontinued therapy, which was mostly due to progressive disease.
Early discontinuation during the first six months occurred in 26.3 %; here, patient outcomes were poor, with a median post-discontinuation survival of 6.9 months. According to multivariate analysis, discontinuation of BTK inhibitors due to toxicity as well as discontinuation within six months were independent predictors of survival. On the other hand, the decision between BTK inhibitor monotherapy and combination therapy did not significantly affect survival, which also applied to the choice of first-generation vs. second-generation agents. The authors concluded that BTK inhibitors are an effective and well-tolerated treatment for long-term use in Chinese patients. Considering the suboptimal outcomes after early discontinuation, these data emphasize the importance of treatment adherence.
German findings for BTK inhibition vs. venetoclax
An analysis by the German CLL Study Group Registry presented at iwCLL 2023 included patients with CLL who were treated with either BTK inhibitors (n = 915) or venetoclax (n = 274) between July 2014 and January 2023 [3]. BTK inhibitors and venetoclax constituted the first-line strategy in 38.5 % and 55.5 %, respectively. Pretreatment mostly consisted of chemoimmunotherapy.
The survival outcomes were comparable irrespective of the type of treatment. Median event-free survival and OS from the first BTK-inhibitor–containing regimen were 23.2 and 85.9 months, respectively; for venetoclax-containing regimens, this was 31.8 and 96.5 months, respectively. Similarly, time to next treatment (TTNT) did not differ, with median TTNT being 68.4 and 63.7 months for the BTK inhibitor and venetoclax groups, respectively.
Acalabrutinib & ibrutinib: differences in CV safety
Safety events and other outcomes were assessed for ibrutinib and acalabrutinib in an observational, retrospective study conducted in Spain [4]. The scientists identified a total of 91 patients at four centers. These had a median age of 73.2 years, and 58.2 % were males. Fifty-eight percent, 31.9 % and 9.9 % were treated in the first, second and subsequent lines, respectively. Acalabrutinib and ibrutinib were used in 27.5 % and 72.5 %, respectively.
No differences resulted across the two agents regarding PFS (p = 0.363) or OS (p = 0.216). However, the second-generation BTK inhibitor acalabrutinib displayed an improved cardiovascular toxicity profile compared to the first-generation agent. Hypertension was found in 14 ibrutinib-treated patients (21.2 %) but in none of those receiving acalabrutinib (p = 0.029). Overall, cardiotoxic events occurred in 24.2 % vs. 12.0 % (p = 0.32). No significant differences were reported in terms of other toxicities including infections, bleeding, hematological events or gastrointestinal events.
The impact of hypertension
Based on data from four Danish registries, Vainer et al. evaluated the impact of hypertension on the treatment and survival outcomes of 6,557 CLL patients [5]. Hypertension is highly prevalent in newly diagnosed CLL and a common adverse event of BTK inhibition. The population had a median age of 71 years, and 46 % had been diagnosed with hypertension.
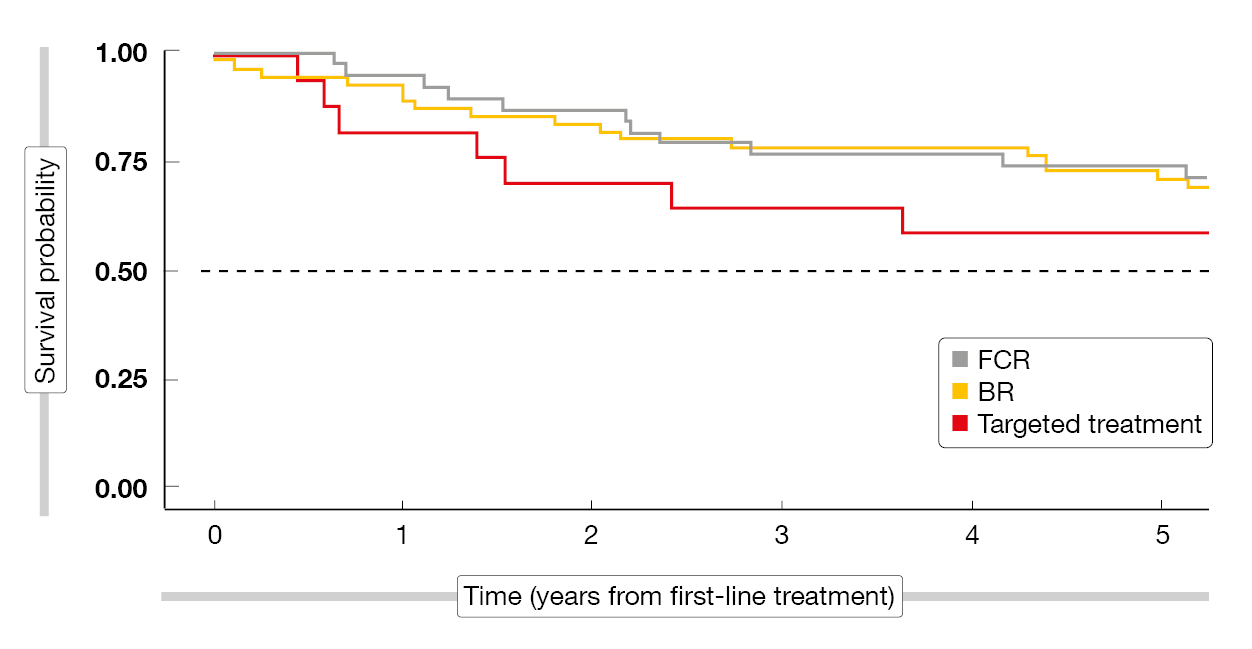
According to this analysis, hypertension was associated with shorter OS, particularly following first-line treatment. The risk of CLL-unrelated and CLL-related death was increased, with patients dying more often from infectious and cardiovascular causes compared to CLL patients without hypertension. Also, time to treatment initiation was comparatively longer. The scientists compared the OS outcomes from the first-line treatment for fludarabine/cyclophosphamide/rituximab, BR, and targeted therapies. In the group with hypertension, patients fared better with chemoimmunotherapy than with targeted agents (Figure 2), whereas there was no difference in those without hypertension. This might be taken into consideration at the time of CLL treatment selection, in addition to the observation that CLL patients with hypertension appear to be more vulnerable to infections.
Figure 2: Overall survival from first-line chemoimmunotherapy (FCR, BR) and targeted agents in CLL patients with hypertension
REFERENCES
- Davids MS et al., Real-world treatment patterns, reasons for discontinuation, and survival outcomes among patients with chronic lymphocytic leukemia/small lymphocytic lymphoma receiving second or later lines of therapy in a contemporary population treated in the United States. iwCLL 2023, poster 1109
- Yan Y et al., Real-world treatment patterns, discontinuation and clinical outcomes in patients with B-cell lymphoproliferative diseases treated with BTK inhibitors in China. iwCLL 2023, poster 1127
- Kutsch N et al., Targeted agents in chronic lymphocytic leukemia: data on outcomes and subsequent therapies of patients observed within the German CLL Study Group registry. iwCLL 2023, poster 1212
- Lopez-Garcia A et al., Safety profile in patients with chronic lymphocytic leukemia undergoing treatment with 1st and 2nd generation Bruton tyrosine kinase inhibitors: multicentric real-world experience. iwCLL 2023, poster 1116
- Vainer N et al., Association between hypertension and overall survival in chronic lymphocytic leukemia. iwCLL 2023, poster 1101
© 2023 Springer-Verlag GmbH, Impressum
More posts
Long-term results and other findings from clinical trials
Long-term results and other findings from clinical trials MURANO: 7-year data and retr
Preface – iwCLL 2023
Preface – iwCLL 2023 © author's own – Kiyomi Mashima MD, PhD, Medical Oncology, CLL C