New insights into BTKi treatment of Waldenström‘s macroglobulinemia
New insights into BTKi treatment of Waldenström’s macroglobulinemia
Waldenström’s macroglobulinemia (WM) is a low-grade non-Hodgkin B-cell lymphoplasmacytic lymphoma, characterized by the accumulation of clonal lymphoplasmacytic cells secreting monoclonal IgM protein in the bone marrow and other organs [1]. WM is a lymphoma accounting for only 1–2 % of all hematologic tumors, with an annual incidence of three to four cases per million people in the USA and Europe, classifying it as a rare disease [2]. Activating mutations in MYD88 and CXCR4 are common in WM and are found in about 90 % and 40 % of patients, respectively [3, 4]. By promoting the proliferation and survival of malignant B-cells, bruton tyrosine kinase (BTK) is a crucial player in the physiopathology of WM [5, 6] and BTK inhibition has been studied as a potential therapeutic approach. Given the efficacy of BTK inhibitors (BTKi), this therapy has rapidly become a new standard-of-care for WM patients [2, 7].
However, research is moving quite fast with the clinical development of new covalent as well as non-covalent BTK inhibitors, BCL2- and CXCR4-antagonists [8, 9].
Effects of BTKi on bone marrow immune microenvironment
BTK, an essential element of the B-cell receptor signaling cascade, plays a crucial role in pathways that regulate tumor microenvironment interactions [6]. Moreover, the bone marrow tumor microenvironment is implicated in WM disease progression and treatment resistance [10]. Thus, Christian et al. investigated immune microenvironmental changes in the bone marrow of BTKi-treated WM patients and assessed their correlation with the molecular profile of patients [11].
The OncomineTM Immune Response Research assay was used to measure the expression of targeted genes. Overall, 400 genes involved in the tumor microenvironment were interrogated with an Ion S5TM sequencing system. In total, 15 patients underwent FFPE trephine bone marrow biopsies before and twelve months after BTKi treatment. Patients’ MYD88 mutational status was correlated with gene expression changes and the AffymetrixTM Transcriptome Analysis Console software was used to perform differential analysis between the two time points.
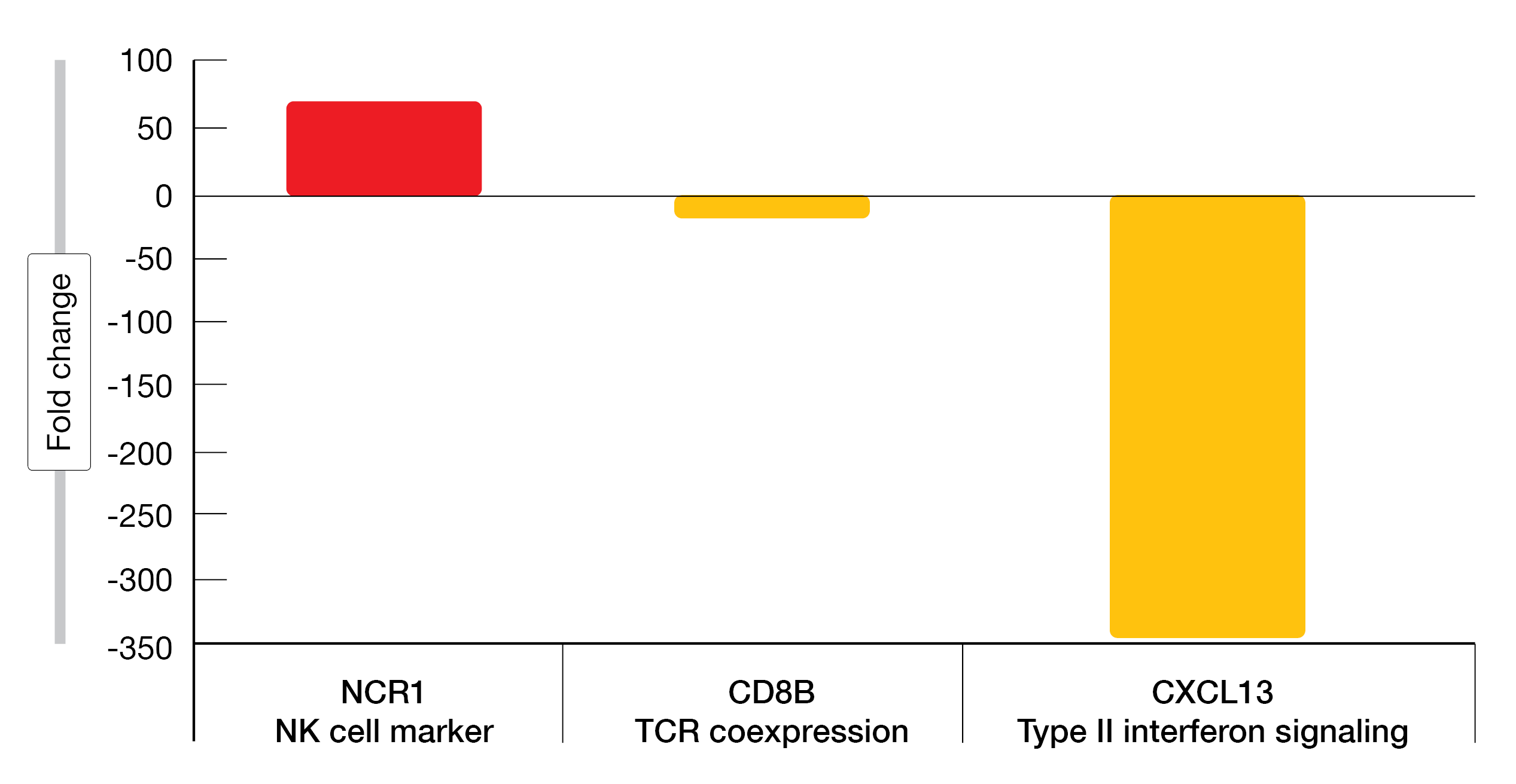
Of the 30 samples plus one control, 14 passed quality control. Nine were positive for the MYD88L265P whereas five were negative. A minor response to treatment or better was achieved in all patients at twelve months. In MYD88L265P positive samples, differences were seen between pre (n = 5) and post (n = 4) treatment in type II interferon signaling, checkpoint and T-cell receptor pathways. NCR1, an NK-cell marker was significantly upregulated and CD8B as well as CXCL13 were significantly downregulated twelve months post BTKi treatment (Figure 1). BTKi treatment types in MYD88L265P positive patients included acalabrutinib (four patients), zanubrutinib (one patient) and ibrutinib (one patient). Of note, CXCL13 downregulation was predominantly observed in patients who received acalabrutinib. Analysis of MYD88L265P negative samples revealed a different gene expression pattern compared to MYD88L265P positive samples with a downregulation of B3GAT1, IL6 and TLR7.
Downregulation of NCR1 gene expression has been shown in patients with B-cell chronic lymphocytic leukemia (B-CLL) but being preserved in patients with small lymphocytic lymphoma [12]. Although ibrutinib was associated with decreased NK-cell cytotoxicity in patients with mantle cell lymphoma (MCL) [13], this report shows for the first time that NCR1, encoding for a cytotoxicity-activating receptor on NK cells, is upregulated upon BKTi treatment in MYD88L265P positive WM patients. The downregulation of CXCL13 after BTKi treatment observed in this study is consistent with that previously described by Vos et al. in WM patients who received ibrutinib [14].
After twelve months of BTKi treatment, this pilot study showed changes in gene expression patterns in the WM immune environment. However, to confirm these findings, a larger cohort with matched samples pre and post BTKi treatment is warranted.
Figure 1: Fold change in gene expression between pre- and post-BTKi treatment for MYD88L265P positive patients (p < 0.03).
BiRD study: real-world data of ibrutinib
Ibrutinib, a first-generation BTKi, has been approved in 2015 as monotherapy or in combination with rituximab for the treatment of adults with WM [15].
The ongoing observational Belgian ibrutinib Real World Data (BirD) study is currently evaluating the effectiveness and safety of ibrutinib in adults with CLL, MCL or WM in routine clinical practice [16]. Progression-free survival (PFS) and overall response rate (ORR) are the primary endpoints; overall survival (OS), time-to-next-treatment (TTNT) and safety are the secondary endpoints. Adverse events (AEs) considered to be related to ibrutinib are collected retrospectively, whereas treatment-emergent AEs (TEAEs) are collected prospectively.
At the time of this third interim analysis, 42 WM patients were included, 39 being analyzed for effectiveness and 41 for safety. Most patients (64.1 %) were male, the median age at initiation of ibrutinib was 67 years (range, 49-89), the median time from diagnosis to ibrutinib initiation was 8.6 years (range, 0.1-20.5), and all evaluable patients (n = 29) carried the MYD88 mutation. IgM-related pathology (63.4 %) was the most common reason for initiating ibrutinib therapy and. most patients received ibrutinib monotherapy (97.6 %). Combination therapy, monotherapy or both had been previously administered in 33.3 %, 15.4 % and 51.3 % of patients, respectively, with more than half of patients (53.8 %) having received at least three lines of prior therapy.
After a median follow-up of 26.9 months, the median PFS reached 50.6 months, while the best ORR attained 87.2 %. The median time to first response was 2.9 months. The median duration of response (mDoR), median OS, and median TTNT were not estimable. While the median duration of ibrutinib treatment was 22.8 months (range, 2.6-55.7), a total of 15 patients (36.6 %) required dose modifications.
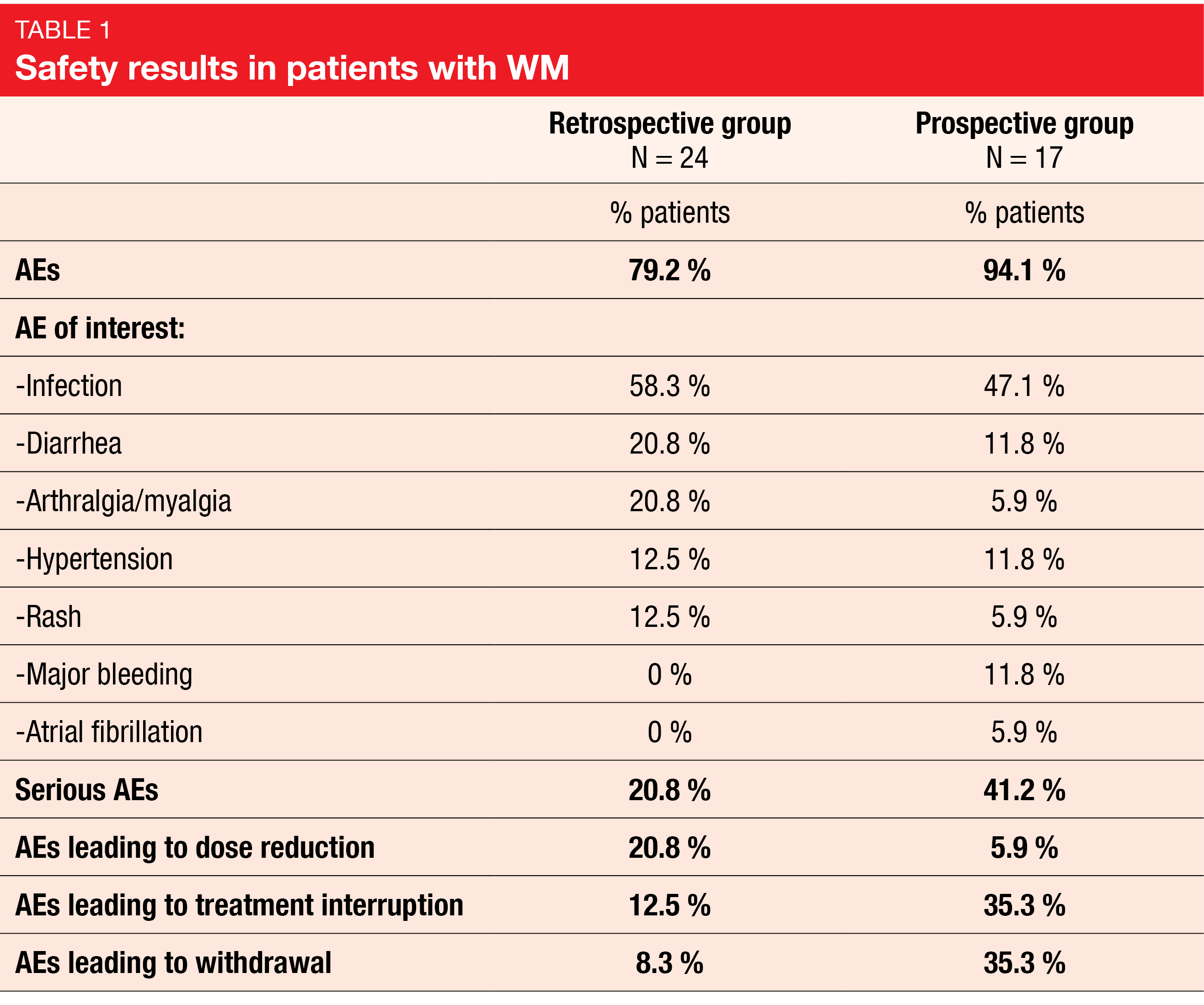
No new safety signals were observed; AEs occurred in 79.2 % of the patients in the retrospective group and 94.1 % of them in the prospective group, whereas serious AEs were reported in 20.8 % and 41.2 %, respectively. In both groups, the AEs of interest (AESIs) included major bleeding, infection, hypertension, atrial fibrillation, arthralgia/myalgia, diarrhea, and rash (Table 1). Among the 41 patients analyzed for safety, 14 (34.1 %) discontinued treatment, five of which were due to disease progression.
This third interim analysis of the BiRD study confirmed the effectiveness and safety of ibrutinib for the treatment of patients with WM in a real word setting. The safety profile of ibrutinib was consistent with that previously described.
Histological transformation after ibrutinib therapy
In WM, histological transformation (HT) to diffuse large B-cell lymphoma (DLBCL) is rare but can occur during the course of the disease or upon treatment and is associated with a poor prognosis [17]. To date, the development of HT has been studied in WM patients treated with chemoimmunotherapy [18-20] but data on its association with newer targeted therapies such as ibrutinib are scarce. From an international multicenter database of 279 patients with transformed WM, 17 patients treated with ibrutinib before developing HT were identified. Their clinical data were reviewed, and a retrospective analysis performed which was presented at this year’s iwWM meeting [21].
At the time of WM diagnosis, patients had a median age of 63 years (range, 36-86); MYD88L265P and CXCR4 mutations were found in 93 % and 57 % of patients respectively. Ibrutinib was administered as primary therapy in two patients only; among the other 15 previously treated patients, the most frequent prior therapies were rituximab (93 %), bendamustine (67 %), proteasome inhibitors (60 %) and rituximab-dexamethasone cyclophosphamide (33 %). At the time of HT, a median of four lines of treatment for WM (range, 1-9), including ibrutinib, had already been administered to patients.
From the diagnosis of WM, HT occurred after a median time of 4.2 years (range, 1–17). Of the ten patients on active treatment with ibrutinib at the time HT, five showed a partial response (PR), two had a very good partial response (VGPR), one had a progressive disease (PD) and one was in complete remission (CR). More than half of the patients (59 %) had extranodal involvement, and five patients presented with central nervous system involvement (3 were detected at the time of HT diagnosis, two of which were on ibrutinib treatment, and two were detected at relapse). Elevated serum LDH levels were observed in 62 % of patients, and 82 % of patients harbored a non-GC phenotype according to the Hans’ algorithm.
Fifteen patients received HT therapy, including R-CHOP in seven patients (47 %), RICE in three patients (20 %), RDHAP in two patients (13 %) and high-dose methotrexate in one patient; a CR was observed in seven patients (50 %), a PR in three (21 %) and a PD in four (29 %). Over time, most patients (86 %) had a disease progression, and the median PFS reached 5.5 months. At the time of last follow-up, 53 % of patients had died with most deaths attributed to disease progression (78 %) or infections (11 %).
Overall, these data show that HT can develop in WM patients even under ibrutinib therapy and that the clinicopathological features appear similar to those previously described in studies on transformed WM. Further studies are needed to better characterize HT in treatment-naïve and in previously treated WM patients as well as to compare HT development after chemoimmunotherapy and novel targeted agents, respectively.
Predictors for ibrutinib response: multi-omic genomics
ons in the MYD88 and CXCR4 genes impact the response to ibrutinib, notably by impacting the time to major response, depth of response and PFS in WM patients [22]. Testing for CXCR4 mutation is challenging – with false negative results in up to two-third of WM patients by next generation sequencing [23]. Moreover, CXCR4 mutations do not fully predict the response activity to BTKi. However, achieving a major response (>PR) at Month 6 is a validated predictor of long-term PFS with ibrutinib [24]. Using a multi-omics approach, easily translatable to the clinical setting, Richardson et al. sought to identify one or more biomarkers predicting response to ibrutinib [25].
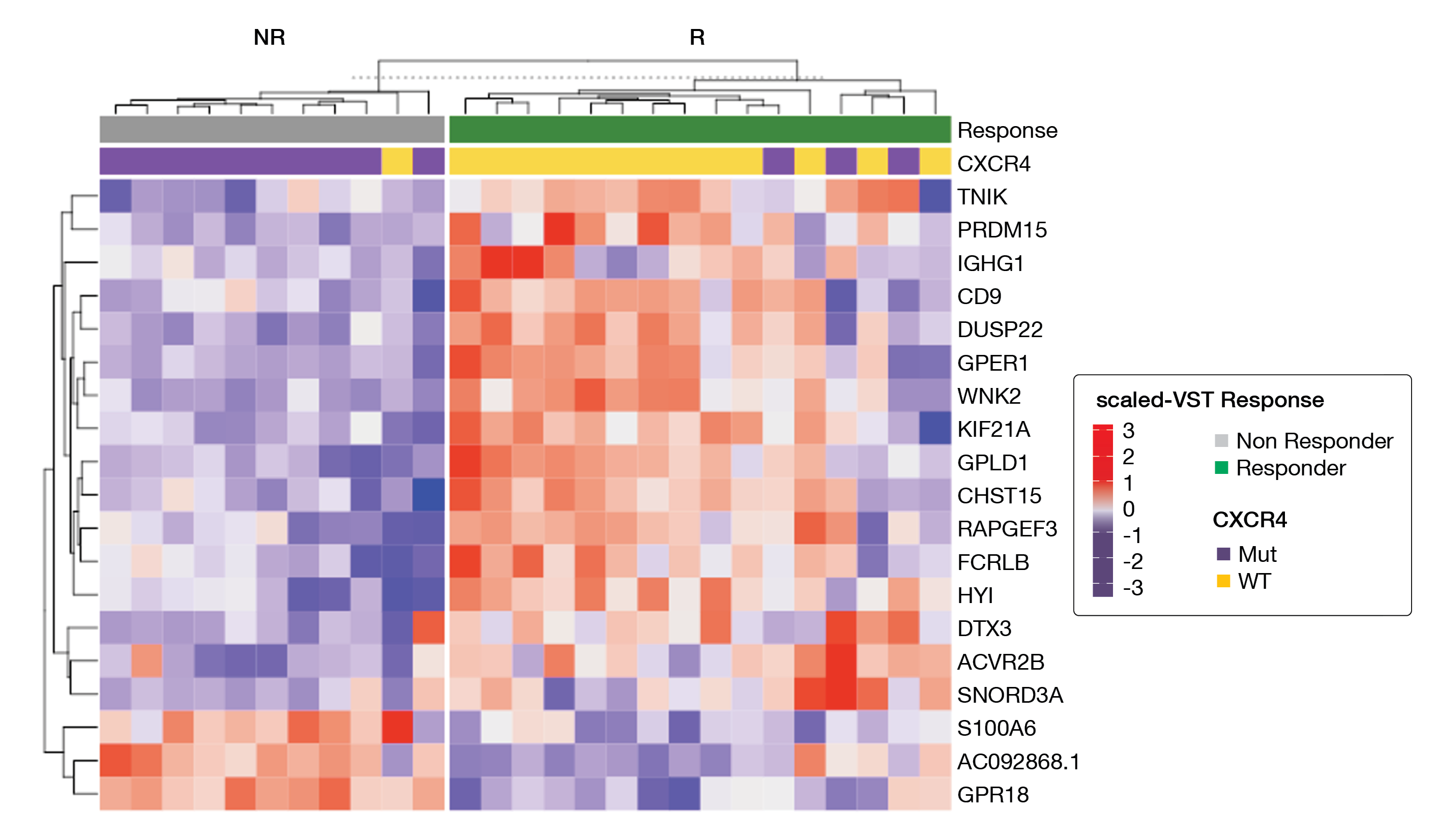
Whole exome and methylome sequencing, RNA-Seq and ATAC-Seq were performed in treatment-naïve, symptomatic patients with MYD88-mutated WM receiving ibrutinib as primary therapy in a prospective clinical trial [26, 27]. At six months, patients with a major response had a longer PFS (median not reached) than patients with a non-major response (64.5 months; p = 0.10). Whole exome sequencing showed that the CXCR4 mutation was the only mutation significantly associated with a major response at six months (p = 0.002). Interestingly, while the CXCR4 mutation was associated with nonresponders, 23 % of the CXCR4-mutated patients were major responders at 6 months. RNA-Seq analysis identified 13 differentially expressed genes between major and non-major responders, including three top hits in major responders (wild-type and mutated CXCR4) namely WNK2 (p = 0.00005), DUSP22 (p = 0.0008) and GPER1 (p = 0.0008). ElasticNet regression analysis using RNA-Seq and ATAC-Seq subjected to 500 bootstraps revealed many regulators of ERK1/2 signaling such as WNK2, DUSP22, GPER1, TNIK, and PRDM15 among the best hits (Figure 2). A strong association was identified between attainment of a major response at six months and the expression of WNK2 (p = 0.0043) as well as DUSP22, GPER1, TNIK and PRDM15 (all p < 0.0001). For these biomarkers, long-term PFS correlated with achieving a major response at six months. Of note, WNK2 expression was validated by immunohistochemistry in bone marrow biopsy samples, too.
By use of a multi-omics approach, robust biomarkers predicting major response to ibrutinib at six months were identified in MYD88-mutated WM patients, including ERK1/2 signaling regulators that further correlated with long-term PFS.
Figure 2: A heatmap showing the scaled variance stabilizing transformation gene levels selected by ElasticNet for baseline expressed genes in MYD88 mutated WM patients who received ibrutinib monotherapy and who attained a major response (green) or no major response (gray) at six months.
Real-world experience with zanubrutinib
Zanubrutinib is a second generation BTKi that has been designed to maximize BTK occupancy and minimize off-target effects [28]. It was recently approved for the treatment of adult WM patients in USA, Canada, and Europe [3, 9, 29]. In a randomized phase III trial comparing the use of zanubrutinib with ibrutinib in WM patients, 28 % of patients who received zanubrutinib achieved a VGPR and the major response rate reached 77 % [30].
The objective of this single-arm expanded access study (NCT04052854) was to provide real-word data on zanubrutinib in treatment-naïve WM patients who were not eligible for chemoimmunotherapy or patients with relapsed/refractory WM [31]. Zanubrutinib was administered at a dose of 320 mg once daily or 160 mg twice daily. Co-primary endpoints included the number of patients enrolled and treated, and the number of enrolling sites, while secondary endpoints included TEAEs, ORR, VGPR or better, PFS and OS. According to the 6th International Workshop on WM [32], response to the treatment was assessed by an investigator at least every sixth month.
From December 2019 to June 2021, 50 WM patients – including 17 treatment-naïve and 33 relapsed/refractory ones – were enrolled in ten US academic and community medical centers. Baseline characteristics included a median age of 72 years, intermediaterisk disease in 54 % of patients and highrisk disease in 40 % of them. Patients with relapsed/refractory disease had a median of two prior therapies. Overall, median treatment exposure was 9.2 months (range, 1.4-20.0).
The safety profile of zanubrutinib was consistent with previous reports. At least one TEAE and one TEAE of special interest was reported in 76 % and 72 % of patients, respectively. Grade ≥3 TEAEs of special interest included hypertension (8 %), infection (8 %), and atrial fibrillation or flutter, neutropenia and second primary malignancy (2 % each).
Among the 41 patients analyzed for efficacy, the ORR was 85.4 % (95 % CI, 70.8-94.4), with a major response rate of 73.2 % (95 % CI, 57.1-85.8). A best overall response of VGPR was achieved in 39.0 % of patients (n = 16; 95 % CI, 24.2-55.5). Of the four patients who had PD, three presented IgM values that met the criteria for partial response before the first response assessment at six months. Because of the short follow-up, PFS and OS were immature and the median was not met.
Real-world experience with zanubrutinib administered as a monotherapy at 320 mg daily in patients with treatment-naïve or relapsed/refractory WM was consistent with the established zanubrutinib profile in WM and other B-cell malignancies.
REFERENCES
- Steingrímsson, V, et al., Epidemiology of Waldenström Macroglobulinemia, ed. T.S. Leblond V, Dimoploulos M, éditeurs. 2017: Springer International Publishing.
- Muñoz, J, et al., Coming of Age for BTK Inhibitor Therapy: A Review of Zanubrutinib in Waldenström Macroglobulinemia. Cells 2022; 11(20): 3287.
- Hunter, ZR, et al., The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014; 123(11): 1637-1646.
- Treon, SP, et al., MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. The New England Journal of Medicine 2012; 367(9): 826-833.
- Gertz, MA, Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. American Journal of Hematology 2019; 94(2): 266-276.
- Pal Singh, S, et al., Role of Bruton’s tyrosine kinase in B cells and malignancies. Molecular Cancer 2018; 17(1): 57.
- Buske, C, et al., Managing Waldenström’s macroglobulinemia with BTK inhibitors. Leukemia 2022.
- Castillo, JJ, et al., What is new in the treatment of Waldenstrom macroglobulinemia? Leukemia 2019; 33(11): 2555-2562.
- Castillo, JJ, et al., Management of Waldenstrom macroglobulinemia in 2020. Hematology Am Soc Hematol Educ Program 2020; 2020(1): 372-379.
- Jalali, S, et al., The Bone Marrow Microenvironment in Waldenström Macroglobulinemia. Hematology/Oncology Clinics of North America 2018; 32(5): 777-786.
- Christian, A, et al., Immune response in Waldenström Macroglobulinaemia patients after BTK inhibition. 2022: iwWM2022, abstract 16.
- Parry, HM, et al., NK cell function is markedly impaired in patients with chronic lymphocytic leukaemia but is preserved in patients with small lymphocytic lymphoma. Oncotarget 2016; 7(42): 68513-68526.
- Flinsenberg, TWH, et al., Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 2020; 105(2): e76-e79.
- Vos, JM, et al., CXCL13 levels are elevated in patients with Waldenström macroglobulinemia, and are predictive of major response to ibrutinib. Haematologica 2017; 102(11): e452-e455.
- Castillo, JJ, et al., Consensus treatment recommendations from the tenth International Workshop for Waldenström Macroglobulinaemia. The Lancet. Haematology 2020; 7(11): e827-e837.
- Snauwaert, S, et al., Effectiveness And Safety Of Ibrutinib In Waldenström’s Macroglobulinemia (WM) In Belgian. 2022: iwWM 2022, abstract 19.
- Castillo, JJ, et al., Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia. American Journal of Hematology 2016; 91(10): 1032-1035.
- Leblond, V, et al., Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2013; 31(3): 301-307.
- Leleu, X, et al., Increased Incidence of Transformation and Myelodysplasia/Acute Leukemia in Patients With Waldenström Macroglobulinemia Treated With Nucleoside Analogs. Journal of Clinical Oncology 2009; 27(2): 250-255.
- Owen, RG, et al., Heterogeneity of histological transformation events in Waldenström’s macroglobulinemia (WM) and related disorders. Clinical Lymphoma, Myeloma & Leukemia 2011; 11(1): 176-179.
- Durot, E, et al., Histological transformation developing after ibrutinib therapy in waldenstrom macroglobulinemia. 2022: iwWM 2022, abstract 21.
- Treon, SP, et al., Genomic Landscape of Waldenström Macroglobulinemia and Its Impact on Treatment Strategies. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2020; 38(11): 1198-1208.
- Gustine, JN, et al., Bone marrow involvement and subclonal diversity impairs detection of mutated CXCR4 by diagnostic next-generation sequencing in Waldenström macroglobulinaemia. British Journal of Haematology 2021; 194(4): 730-733.
- Castillo, JJ, et al., Partial response or better at six months is prognostic of superior progression-free survival in Waldenström macroglobulinaemia patients treated with ibrutinib. British Journal of Haematology 2021; 192(3): 542-550.
- Richardson, K, et al., Identification of robust predictors for ibrutinib response by multi-omic genomics in MYD88 mutated Waldenstrom’s Macroglobulinemia. 2022: iwWM 2022, abstract 24.
- Castillo, JJ, et al., Venetoclax in Previously Treated Waldenstrom Macroglobulinemia. J Clin Oncol 2022; 40(1): 63-71.
- Treon, SP, et al., Ibrutinib Monotherapy in Symptomatic, Treatment-Naïve Patients With Waldenström Macroglobulinemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2018; 36(27): 2755-2761.
- Guo, Y, et al., Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. Journal of Medicinal Chemistry 2019; 62(17): 7923-7940.
- Health Canada Approves BRUKINSA® (Zanubrutinib) for the Treatment of Waldenström’s Macroglobulinemia – BeiGene SSE.
- Tam, CS, et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020; 136(18): 2038-2050.
- de Tute, R, et al., Minimal residual disease (MRD) in Waldenstrom macroglobulinaemia (WM): Depletion of WM-phenotype B-cells is strongly associated with progression following rituximab-based therapy. 2022: iwWM 2022, abstract 25.
- Owen, RG, et al., Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. British Journal of Haematology 2013; 160(2): 171-176.
© 2022 Springer-Verlag GmbH, Impressum
More posts
Emergent BTKi treatments in WM
Emergent BTKi treatments in WM Additionally to ibrutinib, to date the only once-daily B
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
Management of WM patients previously exposed to BTK-inhibitors
Management of WM patients previously exposed to BTK-inhibitors Zanubrutinib in ibrutin
New insights into BTKi treatment of Waldenström‘s macroglobulinemia
New insights into BTKi treatment of Waldenström‘s macroglobulinemia New insights into
Preface – iwWM 2022
Preface – iwWM 2022 © author’s own - Efstathios Kastritis, MD, Plasma Cell Dyscrasia