Detection and treatment of early-stage lung cancer: recent insights
The significance of family history for screening
Given the high incidence and mortality rates of lung cancer in Taiwan and the established association between survival and disease stage at diagnosis, the Taiwanese national early detection program for lung cancer was launched in July 2022 with the aim of increasing the proportion of tumors identified at an early stage using low-dose CT (LDCT). The TALENT study, a domestic study conducted from 2007 to 2011 with 1,102 participants, had already shown that people with a family history of lung cancer are 1.6 times likelier to develop lung cancer than the population without a pertinent family history [1]. Therefore, populations eligible for the early detection program include both heavy smokers (50 to 74 years of age, > 30 pack-years, currently smoking or having quitted < 15 years) and individuals with a family history of lung cancer (males aged 50 to 74 years, females aged 45 to 74 years). Overall, 167 hospitals across the country are participating in the screening program.
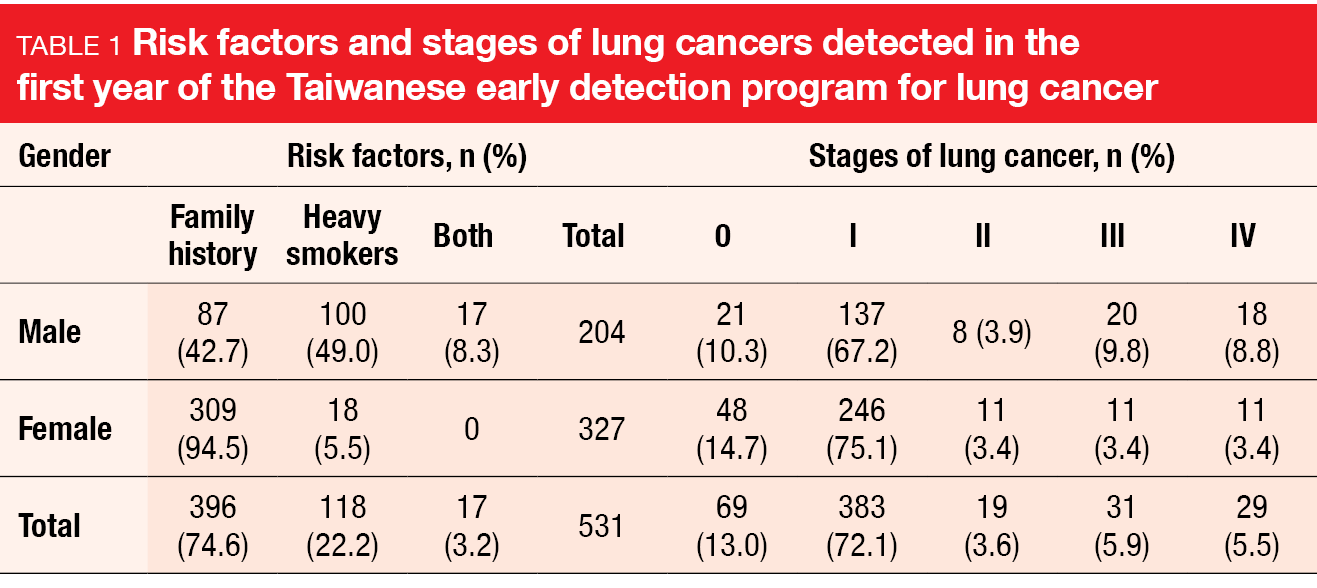
At WCLC 2023, Huang et al. reported the findings obtained during the first year after the start of the program [2]. Almost 50,000 individuals were screened, and 531 lung cancers were detected, which translated into a detection rate of 1.1 %. Among these, 85.1 % were stages 0 or 1 (Table 1). As indicated by the TALENT study, family history was the dominant risk factor. Almost 75 % of lung cancer patients met this criterion, while 22.2 % were heavy smokers; 3.2 % met both criteria. Whereas the positive LDCT screening rate was consistent among populations with a family history, heavy smoking, or both, the lung cancer detection rate was highest in the group with a family history (1.4 vs. 0.6 and 0.9 for heavy smoking and both risk factors, respectively). Likewise, the positive predictive value was twice as high in lung cancer patients with a family history than in the other groups (15.3 vs. 7.1 and 9.8, respectively).
As the authors concluded, these results warrant inviting first-degree relatives of lung cancer patients for screening. Artificial intelligence-based nodule detection algorithms are currently under development to help reduce the reading costs and to raise diagnostic accuracy. Moreover, research is being conducted on the effect of the integration of smoking cessation services in this program, as well as stage changes in newly diagnosed lung cancer cases.
Intra-operative tumor visualization
Screening programs contribute to the increasing number of small lung cancers that require resection. This poses challenges, particularly in the setting of minimally invasive surgery, as palpation cannot be used to identify the tumor. Sub-lobar resection has become the standard procedure for small (< 2 cm) peripheral cancers; here, the margin is critical to prevent recurrence, and node assessment is required to meet the criteria.
A potential solution consists in visualization of the tumor using the fluorescent probe VGT-309, which binds cathepsin in the tumor tissue and associated macrophages. In the first-in-human clinical trial, patients with suspected or proven NSCLC received VGT-309 either on the day before or on the morning of video-assisted thoracic surgery(VATS)/robotic thoracic surgery. Outcomes included the safety of increasing doses and clinically significant events (i.e., index lesion, nodes, additional lesions, unexpected involvement of margins).
According to the results reported at WCLC 2023 by Wright et al., VGT-309 appeared to be a safe and well-tolerated drug [3]. Only one drug-related serious adverse event (AE) occurred, which was asymptomatic transaminitis that recovered spontaneously within 30 days. Increasing doses correlated with increasing mean fluorescence intensity of the tumor. Among 27 participants, 23 were found to have cancer; there was one case of a carcinoid, and three had non-malignant lesions. Tumor visualization was excellent at the maximum dose of 0.32 mg/kg. All of the 12 tumors that were within 10 millimeters of the surface were identified based on fluorescence. Moreover, the data revealed a high tumor-to-background ratio without apparent false-positives.
Clinically significant events were observed in 8 of 20 patients. These included the identification of tumors, positive lymph nodes, and unsuspected pleural metastases that had not been picked up on preoperative imaging. A notable advantage is that VGT-309 works on all commercial VATS and robotic NIR platforms, which renders adaptation unnecessary. To date, further unpublished results have been obtained in a phase II extension as well as a single-center phase II study. A large multi-center phase II trial will be starting soon.
Second primary lung cancers in CALGB 140503
The non-inferiority of sub-lobar resection compared to lobectomy in patients with peripheral NSCLC with a tumor size of ≤ 2 cm and node-negative disease (T1aN0) has been demonstrated by the multicenter phase III CALGB 140503 trial [4]. Both disease-free survival (DFS) and overall survival (OS) were comparable across the two approaches. The analysis presented at WCLC 2023 related to the rate of second primary lung cancers (SPLCs) observed in CALGB 140503 [5]. SPLCs were defined as tumors with a different histology than the initial lung cancer, a new lung cancer which was diagnosed two years after the initial one, or a new tumor diagnosed in a different lobe or segment without intervening lymph nodes or metastases. In the sub-lobar and lobar resection arms, 340 and 357 patients were analyzed, respectively. The objective of the study was the estimation of the overall SPLC rates and the rates observed per patient per year.
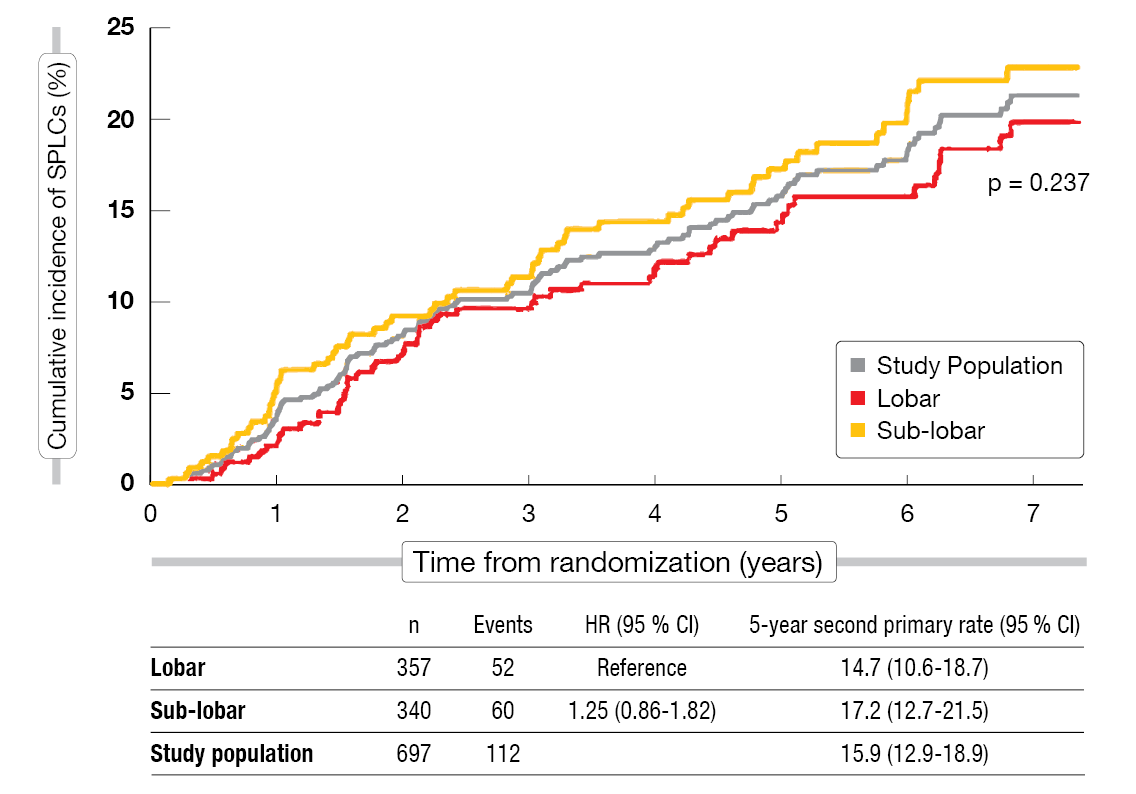
After a follow-up of seven years, the analysis demonstrated a clinically significant SPLC rate, although the difference across the arms was not significant (Figure 1). In the overall study population, 15.9 % of patients had developed SPLCs at 5 years; after sub-lobar resection, this applied to 17.2 %, and after lobar resection, to 14.7 % (p = 0.237). The rates per patient per year were 3.4 %, 3.8 % and 3.1 %, respectively. None of the risk factors for SPLCs evaluated in the univariable and multivariable analysis showed a significant effect, although there was a trend towards current tobacco use. The scientists performed an exploratory analysis that excluded SPLCs occurring during the first two years, as these tend to be more likely due to misclassification. Again, the 5-year SPLC rates did not differ, with 14.9 % overall and 15.9 % and 14.0 % for the sub-lobar and lobar resection groups, respectively (p = 0.466). The rates per patient per year were 1.9 %, 2.1 % and 1.8 %, respectively.
In their conclusion, the authors emphasized that the optimal frequency, duration, and type of surveillance is undefined. Future studies should collect diagnostic information and treatment for SPLCs given the clinical relevant rates. In addition to the projected increase in early-stage NSCLC cases due to increased use of CT screening, improved adjuvant treatments for resected lung cancers are assumed to contribute to the rising incidence of SPLCs.
Figure 1: Cumulative incidence of second primary lung cancer after sub-lobar vs. lobar resection
I-SABR: nivolumab plus radiotherapy
In patients with operable stage I NSCLC (tumor diameter ≤ 3 cm, N0M0), a single-arm prospective trial has demonstrated non-inferiority of stereotactic ablative radiotherapy (SABR) regarding long-term survival compared to VATS [6]. SABR has been implemented as a standard of care. However, the recurrence rates at five years were higher in the SABR arm than in the group undergoing surgery (17.6 % vs. 8 %).
To improve upon these results, an open-label, randomized, phase II trial evaluated the addition of four doses of nivolumab to SABR (50 Gy in 4 fractions or 70 Gy in 10 fractions) in patients with IA-IB (tumor size ≤ 4 cm, N0M0), stage IIA (≤ 5 cm, N0M0), or stage IIB (> 5 cm and ≤ 7 cm, N0M0) NSCLC, including multiple primary tumors. Also, patients with isolated lung parenchymal recurrent or persistent node-negative disease suitable for SABR were enrolled. The control arm received SABR only. In the experimental and control arms, 66 and 75 patients, respectively, were analyzed per protocol. Regarding baseline characteristics, there was a slight imbalance, with patients in the experimental arm harboring comparatively larger tumors (median tumor size, 2.0 vs. 1.7 cm) and a higher percentage of recurrent disease (24 % vs. 16 %). The primary endpoint was the 4-year event-free survival (EFS) rate in the per-protocol population.
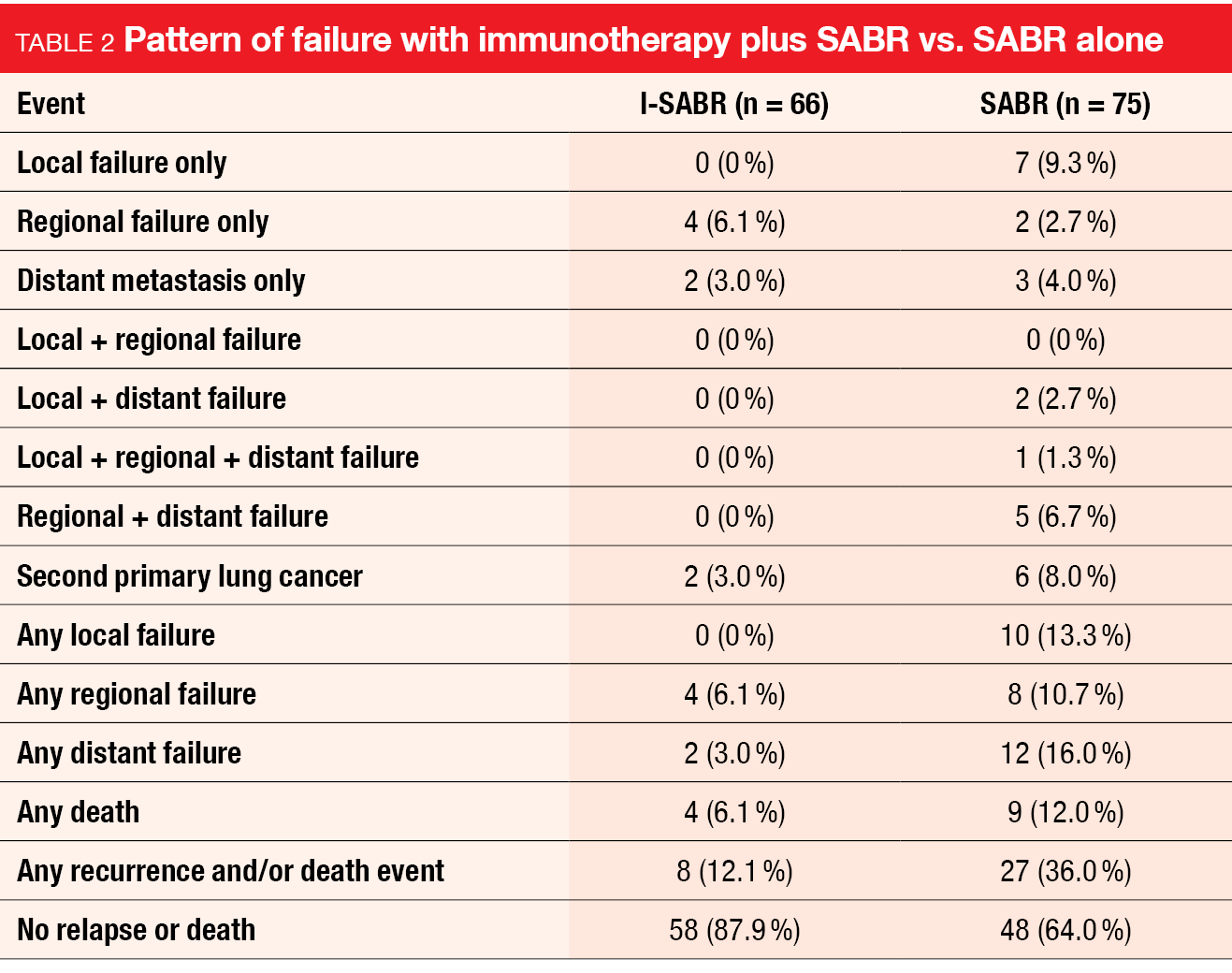
Compared with SABR alone, the addition of adjuvant immunotherapy (I-SABR) significantly improved EFS, with 48-month rates of 77 % vs. 53 % (HR, 0.38; p = 0.0056) [7]. Recurrence and/or death was greatly reduced in the I-SABR arm (12.1 % vs. 36.0 %; Table 2). Also, the patients in the experimental arm showed a lower rate of second primary lung cancers (3.0 % vs. 8.0 %). This might suggest a preventive effect of the immunotherapy combination, although these data are only exploratory. Almost none of all patients experienced in-field failure (0 % vs. 1 %), which underscores the potency of radiotherapy.
Toxicity of the combined regimen was tolerable. Notably, fatigue occurred more commonly with I-SABR than with SABR (grade 2, 7 vs. 1 events; grade 3, 2 vs. 0 events). Grade 2 pneumonitis emerged in 2 vs. 1 patients, with no individual developing grade 3 pneumonitis. Overall, no grade 4 or 5 AEs were observed in the study. An ongoing exploratory predictive analysis using radiomic artificial intelligence modeling is attempting to identify patients in need of immunotherapy. According to the authors, I-SABR might be a treatment option in patients with early-stage, treatment-naïve NSCLC or parenchymal, node-negative recurrent lung cancer. A phase III study is required to establish this approach in routine care.
Perioperative durvalumab in EGFR-mutant disease
AEGEAN was the first phase III study to evaluate the perioperative use of the PD-L1 inhibitor durvalumab in addition to neoadjuvant chemotherapy for patients with resectable NSCLC (stage IIA-IIIB[N2]) [8]. Durvalumab 1,500 mg plus platinum-based chemotherapy Q3W for four cycles was tested against placebo plus chemotherapy prior to lobectomy, sleeve resection, or bilobectomy. After surgery, patients in the experimental arm continued to receive durvalumab consolidation Q4W for 12 cycles, while those in the control arm were treated with placebo. The combined approach led to improvements in EFS (HR, 0.68; p = 0.003902) and the pathological complete response (pCR) rate (p = 0.000036). As enrollment started prior to the emergence of evidence indicating that patients with EGFR/ALK aberrations might have a limited response to immunotherapy, the protocol was amended in 2021 to exclude this population.
At WCLC, He et al. reported the outcomes for 51 patients with EGFR mutations who entered the AEGEAN study between 2019 and 2021 before the protocol amendment [9]. Among these, 26 and 25 were treated with durvalumab and placebo, respectively. There were some imbalances in baseline characteristics, such as higher rates of males and Asian patients in the placebo arm than in the durvalumab arm. Exon 19 deletions were present in 53.8 % vs. 36.0 % in the durvalumab and placebo groups, respectively, and L858R mutations were found in 11.5 % vs. 16.0 %. While only 3.8 % of those in the experimental arm had EGFR mutations categorized as “other”, this was the case in 24.0 % in the control arm.
The subgroup of patients with EGFR-mutated NSCLC, in contrast to the modified ITT (mITT) population, did not derive a clear benefit from perioperative durvalumab in addition to neoadjuvant chemotherapy. After a median follow-up of 16.6 months, median EFS did not differ significantly (HR, 0.86), with 24-month EFS rates of 59.3 % vs. 44.9 %. The difference in pCR rates was low at 3.8 % (3.8 % vs. 0.0 %), whereas this was 13.0 % in the mITT population (17.2 % vs. 4.3 %). Similarly, major pathological responses occurred in 7.7 % vs. 4.0 % in the EGFR-mutant group and in 33.3 % vs. 12.3 % in the mITT population. The toxicity observed for patients with EGFR mutations was manageable.
These findings should be interpreted with caution due to the small numbers of patients with EGFR-mutant disease and wide 95 % confidence intervals. As the authors noted, EGFR testing should be considered prior to the initiation of neoadjuvant therapy in light of the robust clinical benefit demonstrated for the adjuvant administration of the EGFR TKI osimertinib in the phase III ADAURA trial after resection of EGFR-mutated lung cancer [10].
PACIFIC-R: outcomes by EGFR mutational status
Consolidation treatment with durvalumab for up to 12 months has been established as a global standard of care for patients with unresectable, stage III NSCLC after chemoradiotherapy based on the placebo-controlled, phase III PACIFIC trial, with the OS and PFS benefits being maintained over time [11]. The ongoing international, observational PACIFIC-R study has confirmed the real-world effectiveness of this regimen [12]. PACIFIC-R is being conducted as a retrospective review of medical records for patients from the PACIFIC early access program. Due to uncertainty regarding the efficacy of consolidation immunotherapy in patients with EGFR-mutated lung cancer, Peters et al. explored the outcomes in PACIFIC-R according to EGFR mutational status [13].
Within the full analysis set of 1,154 patients recruited across eight European countries plus Australia and Israel, the EGFR mutation status was known in 466 individuals (40.4 %). While 44 (9.4 %) had EGFR-mutated NSCLC, 422 (90.6 %) had EGFR-wildtype disease. Clinical characteristics were generally similar between the mutated and wildtype groups, although a lower proportion of EGFR-mutated patients was aged < 70 years, had a history of smoking, and had a performance status of 0. In approximately 75 % of cases, PD-L1 expression ≥ 1 % was present on tumor cells irrespective of EGFR status.
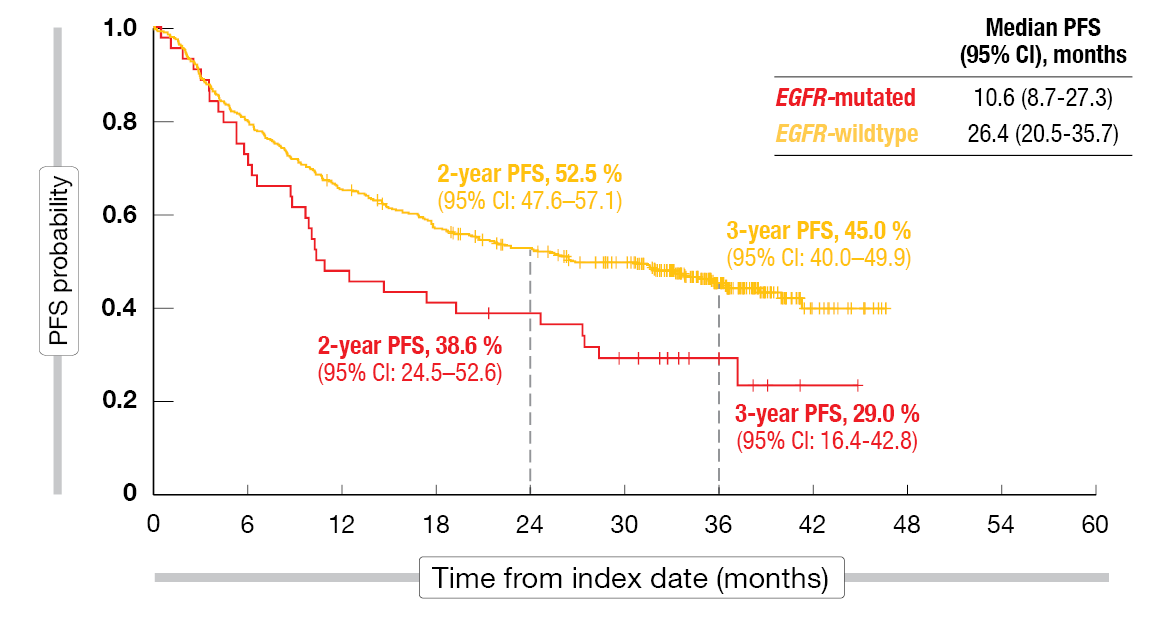
Real-world PFS in PACIFIC-R was shown to be shorter among patients with EGFR-mutated NSCLC than in those with EGFR wildtype (10.6 vs. 26.4 months; Figure 2). The 3-year PFS rates were 29.0 % vs. 45.0 %. At the same time, OS was comparable across the groups (46.3 months vs. not reached), with 3-year OS rates of 64.9 % vs. 67.9 %. With regard to time to death or distant metastasis, the EGFR-mutated group again fared worse than the EGFR wildtype group (24.7 vs. 38.6 months; 3-year rates, 35.3 % vs. 52.9 %). Also, time to the first subsequent treatment was shorter among patients with EGFR-mutant disease (16.7 vs. 43.1 months; 3-year rates, 33.3 % vs. 55.4 %). Almost half of patients with EGFR-mutated NSCLC experienced distant metastasis. The PFS and OS outcomes with durvalumab were broadly comparable to those obtained in the EGFR-mutated subgroup treated with durvalumab in the PACIFIC trial (n = 24) [14, 15]. However, the authors cautioned that small sample sizes and the retrospective nature of the PACIFIC-R study limit the interpretation of these findings.
Figure 2: Investigator-assessed progression-free survival by EGFR status in PACIFIC-R
Osimertinib vs. durvalumab consolidation
A retrospective, multicenter analysis reported at WCLC 2023 has revealed superiority of consolidation EGFR TKI treatment with osimertinib compared to durvalumab and observation following chemoradiation for stage III, locally advanced, unresectable, EGFR-mutant NSCLC [16]. These patients had received ≥ 2 cycles of platinum-based concurrent chemoradiation and had shown no disease progression at the time of initiation of consolidation therapy. Within the total group of 136 individuals treated at 24 institutions around the globe, 33 and 56 received osimertinib and durvalumab consolidation, respectively, while 47 were observed. DFS and OS constituted the coprimary endpoints.
Osimertinib consolidation induced a significant DFS benefit compared to both durvalumab consolidation and observation (p < 0.0001). At 24 months, the DFS rate was 86 % for osimertinib, while the respective rates for durvalumab and observation were markedly lower at 31 % and 29 %, respectively. Regarding the endpoint of central nervous system DFS, however, osimertinib showed a lower 24-month rate (6.7 %) than durvalumab and observation (17 % and 11 %, respectively). No difference across the three groups was noted in terms of OS (p = 0.31). This might be due to the subsequent EGFR TKI therapy that was administered to substantial proportions of patients in the durvalumab and observation groups. Another factor possibly explaining the lack of difference in OS is the limited follow-up time.
The analysis revealed no unanticipated safety signals. As expected, pneumonitis was more common with durvalumab (any grade, 25 %; grade ≥ 3, 13 %) than with osimertinib (any grade, 15 %; grade ≥ 3, 3 %). Among 37 patients who received EGFR TKIs after durvalumab, 38 % experienced treatment-related AEs on TKI therapy. Five of these developed pneumonitis, including two grade ≥ 3 cases, and five had diarrhea/colitis including one grade ≥ 3 event. As the authors emphasized in their summary, prospective data are required to confirm these findings. The ongoing phase III LAURA study is assessing the efficacy and safety of maintenance osimertinib in patients with unresectable, stage III, EGFR-mutated NSCLC who have not progressed after chemoradiotherapy (NCT03521154).
REFERENCES
- Yang P, National Lung cancer screening program in Tawain: The TALENT study. J Thorac Oncol 2021; 16(3, Supplement): S58
- Huang KP et al., The early detection program for lung cancer in Taiwan. WCLC 2023, abstract PL03.04
- Wright GM et al., Results from a phase II trial of VGT-309, a tumor-activated fluorescent molecule for the intra-operative identification of lung cancer. WCLC 2023, abstract MA11.06
- Altorki N et al., Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med 2023; 388(6): 489-498
- Stinchcombe TE et al., Second primary lung cancer from Cancer and Leukemia Group B (CALGB) 140503 (Alliance) trial of lobar versus sub-lobar resection for T1aN0 non-small cell lung cancer. WCLC 2023, abstract OA12.03
- Chang JY et al., Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol 2021; 22(10): 1448-1457
- Chang JY et al., Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative NSCLC: an open-label, randomized, phase 2 trial. WCLC 2023, abstract OA12.04
- Heymach JV et al., AEGEAN: a phase 3 trial of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res 2023; 83 (8_Supplement): CT005
- He J et al., Neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in resectable EGFR-mutated NSCLC (AEGEAN). WCLC 2023, abstract OA12.06
- Tsuboi M et al., Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med 2023; 389(2): 137-147
- Spigel DR et al., Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 2022; 40(12): 1301-1311
- Girard N et al., Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: Findings from the PACIFIC-R study. J Thorac Oncol 2023; 18(2): 181-193
- Peters S et al., Real-world outcomes with durvalumab after chemoradiotherapy in unresectable stage III EGFR-mutated NSCLC (PACIFIC-R). WCLC 2023, abstract OA17.03
- Naidoo J et al., Brief report: Durvalumab after chemoradiotherapy in unresectable stage III EGFR-mutant NSCLC: A post hoc subgroup analysis from PACIFIC. J Thorac Oncol 2023; 18(5): 657-663
- Naidoo J et al., Durvalumab after chemoradiotherapy in unresectable, stage III, EGFR mutation-positive NSCLC: A post hoc subgroup analysis from PACIFIC. J Clin Oncol 40, 2022 (suppl 16; abstr 8541)
- Nassar AH et al., Consolidation EGFR-tyrosine kinase inhibitor vs. durvalumab vs. observation in unresectable EGFR-mutant stage III NSCLC. WCLC 2023, abstract MA16.11
© 2023 Springer-Verlag GmbH, Impressum
More posts
MARS 2: no benefit of decortication in early-stage malignant mesothelioma
MARS 2: no benefit of decortication in early-stage malignant mesothelioma Surgery in th
Trop-2–directed ADCs in conjunction with immunotherapy: EVOKE-02 and TROPION-Lung04
Trop-2–directed ADCs in conjunction with immunotherapy: EVOKE-02 and TROPION-Lung04
Refining first-line regimens for extensive-stage small-cell lung cancer
Refining first-line regimens for extensive-stage small-cell lung cancer Benmels
Improved anti-EGFR strategies and other targeted innovations
Improved anti-EGFR strategies and other targeted innovations FLAURA2: first-lin
Detection and treatment of early-stage lung cancer: recent insights
Detection and treatment of early-stage lung cancer: recent insights The signifi
Preface – WCLC 2023
Preface – WCLC 2023 © private – Navneet Singh, MD DM FRCP FASCO, Professor of Pulmona