Refining first-line regimens for extensive-stage small-cell lung cancer
Benmelstobart plus anlotinib and chemotherapy
Improving long-term survival remains an unmet need in the setting of extensive-stage small-cell lung cancer (ES-SCLC). This might be attributed at least in part to the complexity of the tumor microenvironment, which is characterized by immunosuppression, angiogenesis and vascularization [1, 2]. It was hypothesized that microenvironment reprogramming and tumor vessel normalization could promote immune cell infiltration, thus improving synergy with immunotherapy [1-3]. The novel PD-L1 inhibitor benmelstobart combined with the anti-angiogenic multi-target tyrosine kinase inhibitor anlotinib was tested by the randomized, double-blind, phase III ETER701 trial in addition to standard chemotherapy in patients with ES-SCLC who had not received any prior systemic therapy. At WCLC 2023, Cheng et al. reported findings for 246 patients treated with benmelstobart 1,200 mg on day 1 plus anlotinib 12 mg on days 1–14 and etoposide/carboplatin for four 21-day cycles [4]. The control group contained 247 patients who received double placebo instead of benmelstobart and anlotinib in addition to etoposide/carboplatin. After the induction phase, maintenance consisted of benmelstobart plus anlotinib in the experimental arm and placebo in the control arm. Overall survival (OS) and progression-free survival (PFS) constituted the primary endpoints. ETER701 has a third study arm testing placebo plus anlotinib and chemotherapy, for which no results were reported.
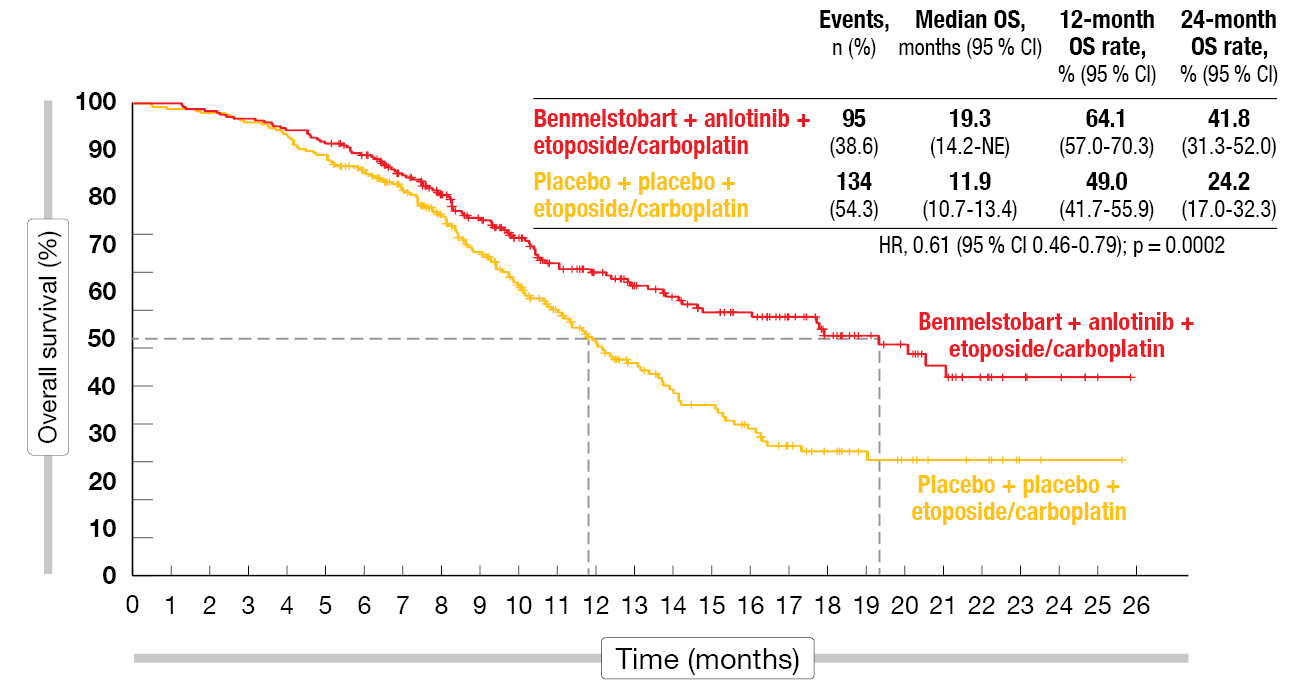
Indeed, median OS was significantly prolonged with the four-drug regimen as compared to chemotherapy alone (19.3 vs. 11.9 months; HR, 0.61; p = 0.0002; Figure). At 12 months, the OS rates were 64.1 % vs. 49.0 %, and at 24 months, 41.8 % vs. 24.2 %. For PFS, the treatment benefit was equally impressive, with median PFS of 6.9 vs. 4.2 months (HR, 0.32; p < 0.0001) and 12-month PFS rates of 27.9 % vs. 2.3 %. All of the subgroups favored benmelstobart plus anlotinib and chemotherapy with regard to both OS and PFS. Also, duration of response was significantly improved in the experimental arm (5.8 vs. 3.1 months; HR, 0.31; p < 0.0001), as was the confirmed objective response (81.3 % vs. 66.8 %; p = 0.0001). For disease control, the analysis yielded no significant difference (90.7 % vs. 87 %).
The safety profile of the four-drug combination proved tolerable and manageable. Cytopenia was the most common toxicity in both study arms. Any-grade immune-related adverse events (irAEs) were observed in 42.7 % (grade ≥ 3, 16.7 %) with benmelstobart plus anlotinib and chemotherapy vs. 19.1 % with chemotherapy only (grade ≥ 3, 6.9 %). In the experimental arm, irAEs necessitated dose reductions and discontinuation in 6.5 % and 8.1 %, respectively. Death due to irAEs occurred in five cases (2.0 %) vs. one case in the control arm (0.4 %). The authors concluded that the addition of the anti-angiogenic agent anlotinib to immunochemotherapy in the first-line treatment of ES-SCLC resulted in the historically longest PFS and OS, thus supporting this regimen as a new treatment option.
Figure: Benmelstobart plus anlotinib and chemotherapy vs. placebo plus chemotherapy: overall survival
Addition of tislelizumab: RATIONALE-312
Phase II data have shown promising activity of the anti–PD-1 antibody tislelizumab in combination with chemotherapy in patients with untreated ES-SCLC [5]. The randomized, double-blind, placebo-controlled, phase III RATIONALE-312 study was conducted to compare tislelizumab 200 mg on day 1 plus carboplatin or cisplatin and etoposide Q3W (n = 227) with placebo plus chemotherapy (n = 230) in the untreated ES-SCLC setting. After four cycles of induction treatment, the patients in the experimental and control arms received tislelizumab 200 mg Q3W and placebo, respectively, as maintenance. The majority of patients in both arms had ≥ 3 metastatic sites.
RATIONALE-312 met its primary endpoint, demonstrating a statistically significant and clinically meaningful OS improvement for the addition of tislelizumab to chemotherapy compared with chemotherapy alone (15.5 vs. 13.5 months; HR, 0.75; p = 0.0035) [6]. At 24 months, 33.2 % vs. 22.4 % of patients were alive. The risk of progression or death was reduced by 37 % in the experimental arm, with 12-month PFS rates of 20.7 % vs. 4.5 % (HR, 0.63; p < 0.0001). All of the predefined subgroups benefited from the combined approach in terms of both OS and PFS. This was accompanied by an increase in the overall response rate (ORR; 68.3 % vs. 61.7 %) and more durable responses (median duration of response, 4.3 vs. 3.7 months).
Tislelizumab plus chemotherapy showed a manageable safety profile. The combined approach did not give rise to higher rates of the most common AEs that included anemia, alopecia, neutropenia, decreased white blood cell count, thrombocytopenia, and nausea. Treatment-emergent AEs leading to discontinuation occurred in 13.2 % vs. 3.1 %. Immune-mediated AEs were observed in 38.3 % vs. 17.9 % and infusion-related reactions in 3.5 % vs. 2.2 %. The most common immune-mediated AEs on tislelizumab-based treatment included hypothyroidism (13.7 %), rash (13.2 %), and hyperthyroidism (5.7 %). As the authors concluded, RATIONALE-312 confirms that tislelizumab in combination with chemotherapy can improve OS in ES-SCLC, adding supporting evidence for the first-line use of PD-1 inhibitors in this setting.
IMbrella A: long-term survivors from IMpower133
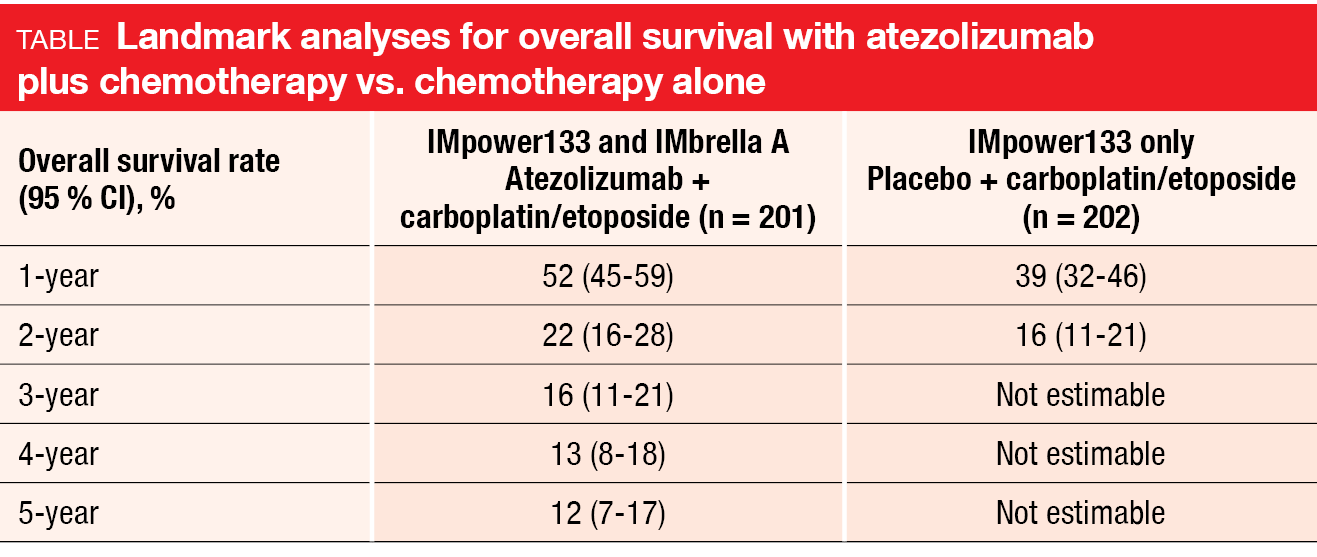
The PD-L1 inhibitor atezolizumab plus carboplatin and etoposide has been established as a first-line treatment standard for patients with ES-SCLC by the double-blind, placebo-controlled, phase III IMpower133 trial [7]. Four 21-day cycles were followed by atezolizumab maintenance. Compared to placebo plus chemotherapy, the atezolizumab-based approach led to improvement of both OS (12.3 vs. 10.3 months; HR, 0.70; p = 0.007) and PFS (5.2 vs. 4.3 months; HR, 0.77; p = 0.02). Updated results showed continued OS benefit [8]. The patients included in the experimental arm of IMpower133 were eligible to roll over to the open-label, non-randomized, multicenter, phase IV IMbrella A study if they continued to receive atezolizumab at study closure or were in survival follow-up. Between December 2019 and July 2020, 18 patients entered the extension and long-term observational study. Compared to all 201 patients randomized to atezolizumab plus carboplatin/etoposide in IMpower133, the IMbrella A cohort was younger, had a better performance status and was less likely to have liver metastasis. Liu et al. presented a merged analysis from IMpower133 and IMbrella A with a clinical cutoff date of 16 March 2023 [9]. This is the first report of 5-year survival outcomes for ES-SCLC patients who received first-line immunotherapy plus chemotherapy.
The 5-year OS rate obtained with atezolizumab plus chemotherapy was 12 %, with the landmark analyses suggesting a plateau in the survival curve (Table). These data compare favorably with historical 5-year OS rates of approximately 2 % in ES-SCLC patients treated with chemotherapy alone [10-12]. All patients who rolled over to IMbrella A achieved either complete or partial responses; no patients with stable disease rolled over. Eleven patients remained alive at 5 years, six of whom continued to receive atezolizumab.
The scientists assessed the distribution of transcriptional subtypes as defined by Gay et al. [13] in patients who were alive and on-study in IMbrella A after 5 years of follow-up. Baseline RNAseq data were available for seven of eleven patients. According to this analysis, four patients had the SCLC-N (NEUROD1-driven) subtype, two the SCLC-I (inflamed) subtype and one the SCLC-A (ASCL1-driven) subtype. In the full RNAseq-evaluable IMpower133 cohort (n = 271), the SCLC-A subtype had been the most common one. Conclusions cannot be drawn due to the small sample size, although it appears that transcriptional subtypes alone do not predict long-term survival.
The final safety analysis for patients from IMpower133 and IMbrella A was consistent with the primary analysis. There was a low incidence of serious AEs and AEs of special interest; only one late-onset immune-related toxicity occurred, which was hypothyroidism grade 2. Taken together, these results demonstrated the potential for a durable survival benefit with atezolizumab plus chemotherapy.
BI 764532 in DLL3-positive tumors
The novel DLL3-targeting T cell engager BI 764532 has been designed to bind to both CD3 on T cells and the antigen DLL3 that is expressed on neuroendocrine carcinomas [14]. Thus, T cells are redirected to the tumor where they induce cell death. Wermke et al. presented findings from the first-in-human phase I dose escalation trial investigating BI 764532 in patients with advanced, DLL3-positive SCLC or neuroendocrine carcinoma (NEC) [15]. These had either progressed after available standard therapies, including ≥ 1 line of platinum-based chemotherapy for SCLC patients, or were ineligible for them. In the group of 107 patients evaluated in the analysis, 53 % had SCLC, while 38 % and 8 % had extrapulmonary NEC and large-cell NEC of the lung (LCNEC), respectively. One third had been treated with ≥ 3 lines, and almost half had received prior immune checkpoint inhibition.
BI 764532 was found to show an acceptable and manageable safety profile at clinically efficacious dose levels in patients with SCLC and LCNEC. Cytokine release syndrome (CRS) occurred as the most common AE (all grades, 48 %), although these events were mostly grade 1 and 2 and usually emerged during initial drug administrations. They were managed with supportive care, corticosteroids and/or anti–IL-6R antibodies. Further AEs included asthenia (32 %), dysgeusia (27 %), constipation (27 %), transient decreases in lymphocyte counts (24 %), and nausea (23 %). Pyrexia, which emerged in 17 %, was restricted to grade 1 and 2 events. At 6 %, the treatment discontinuation rate due to treatment-related AEs was low. Dose-limiting toxicities comprised CRS grade 3–4, confusional state grade 3, infusion-related reaction grade 2, and nervous system disorder grade 3. All of these were reversible, and the maximum tolerated dose had not been reached at the time of the analysis.
With respect to efficacy, the assessments demonstrated tumor shrinkage at doses ≥ 90 µg/kg, with ORRs of 26 % and 60 % in the SCLC and LCNEC groups, respectively. Disease control was achieved in 51 % and 100 %, respectively. Responses appeared to be durable, although it was too early to assess the median duration of response. Further dose optimization is ongoing.
REFERENCES
- Li T, Qiao T, Unraveling tumor microenvironment of small-cell lung cancer: Implications for immunotherapy. Semin Cancer Biol 2022; 86(Pt 2): 117-125
- Chan JM et al., Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell 2021; 39(11): 1479-1496
- Augustin HG, Koh GY, Antiangiogenesis: Vessel regression, vessel normalization, or both? Cancer Res 2022; 82(1): 15-17
- Cheng Y et al., Benmelstobart with anlotinib plus chemotherapy as first-line therapy for ES-SCLC: a randomized, double-blind, phase III trial (ETER701). WCLC 2023, abstract OA01.03
- Wang Z et al., A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in Chinese patients. Lung Cancer 2020; 147: 259-268
- Cheng Y et al., First-line chemotherapy with or without tislelizumab for extensive-stage small cell lung cancer: RATIONALE-312 phase 3 study. WCLC 2023, abstract OA01.06
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379(23): 2220-2229
- Liu SV et al., Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021; 39(6): 619-630
- Liu SV et al., Five-year survival in patients with ES-SCLC treated with atezolizumab in IMpower133: IMbrella A extension study results. WCLC 2023, abstract OA01.04
- Zhang S, Cheng Y, Immunotherapy for extensive-stage small-cell lung cancer: current landscape and future perspectives. Front Oncol 2023; 13: 1142081
- Arriola E et al., Prognostic value of clinical staging according to TNM in patients with SCLC: A real-world surveillance epidemiology and end-results database analysis. JTO Clin Res Rep 2022; 3(1): 100266
- Schabath MB et al., Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer 2014; 86(1): 14-21
- Gay CM et al., Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021; 39(3): 346-360.e.7
- Hipp S et al., A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 2020; 26(19): 5258-5268
- Wermke M et al., Phase I dose escalation trial of the DLL3/CD3 IgG-like T cell engager BI 764532 in patients with DLL3+ tumors: focus on SCLC and LCNEC. WCLC 2023, abstract OA01.05
© 2023 Springer-Verlag GmbH, Impressum
More posts
MARS 2: no benefit of decortication in early-stage malignant mesothelioma
MARS 2: no benefit of decortication in early-stage malignant mesothelioma Surgery in th
Preface – WCLC 2023
Preface – WCLC 2023 © private – Navneet Singh, MD DM FRCP FASCO, Professor of Pulmona