MARS 2: no benefit of decortication in early-stage malignant mesothelioma
Surgery in the form of maximum cytoreduction is recommended to reduce all visible disease in the setting of resectable malignant pleural mesothelioma [1-4]. Extra-pleural pleurectomy/decortication has been established as the predominant approach offered worldwide. However, this approach has never been evaluated in a randomized controlled trial. Therefore, the multicenter, randomized, controlled MARS 2 trial was initiated to compare the clinical efficacy and cost-effectiveness of (extended) pleurectomy/decortication plus chemotherapy with chemotherapy alone for patients with resectable pleural mesothelioma confined to one hemi-thorax. After two cycles of platinum plus pemetrexed, patients underwent computed tomography to confirm resectability and were randomized to either (extended) pleurectomy/decortication followed by up to four further cycles of platinum plus pemetrexed (n = 169), or chemotherapy alone (n = 166).
Eighty-six percent of patients in each arm had epithelioid mesothelioma. In 88.5 % of cases, extended pleurectomy/decortication was performed, while 8.3 % and 1.9 % underwent pleurectomy/decortication and partial pleurectomy, respectively. R0 resection without residual tumor tissue was achieved in 3.2 %. The proportion of patients completing six chemotherapy cycles was lower in the surgery group than in the chemotherapy-only group (39.1 % vs. 56.0 %). Also, fewer patients who underwent decortication were able to receive subsequent immunotherapy or other agents known to improve survival (21.9 % vs. 38.6 %). Overall survival (OS) constituted the primary outcome.
More serious AEs and lower quality of life
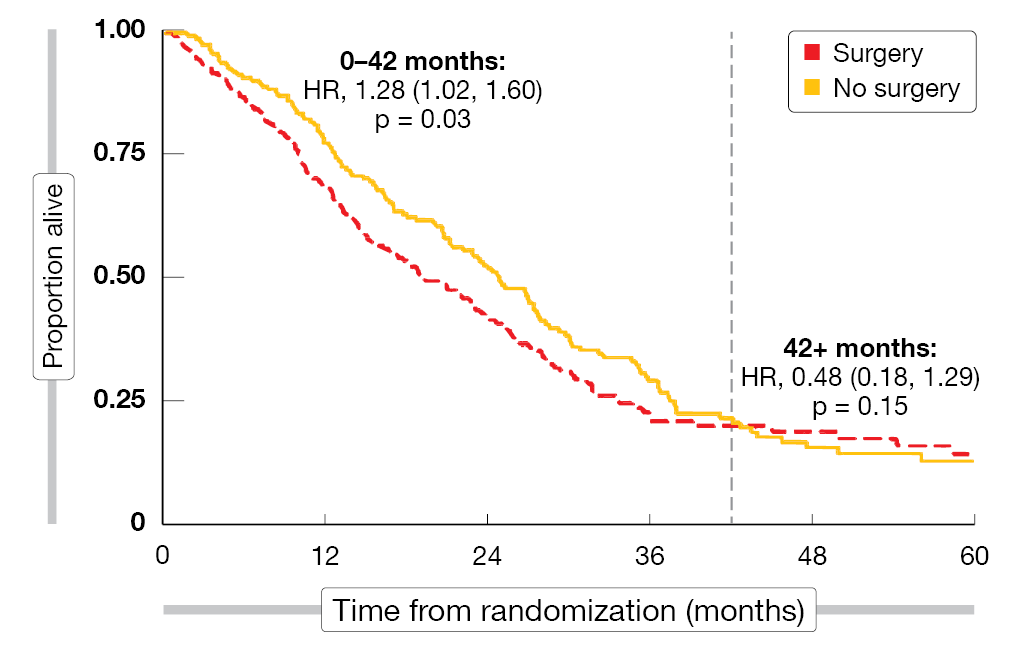
According to the analysis presented at WCLC 2023, extended pleurectomy/decortication did not confer any survival advantage [5]. On the contrary, patients who underwent surgery had shorter OS than those receiving chemotherapy alone, with a 28 % increase in the risk of death (Figure). Even if the analysis was confined to patients with epithelioid histology that showed longer OS than the non-epithelioid subgroup, the mortality risk was increased by 12 %. In the control arm, survival was excellent; due to this, the trial had to be extended by six months to obtain the required number of deaths. No difference resulted for progression-free survival across the arms (HR, 0.90; p = 0.33).
The in-hospital 90-day mortality rate after surgery was 8.9 %. With respect to grade ≥ 3 adverse events, the risk was 3.6-fold higher in the surgically treated arm than in the chemotherapy arm (p < 0.001). This difference centered around cardiac disorders, infections, repeat interventions and events classified as any respiratory, thoracic or mediastinal disorders. Sensitivity analyses consistently favored the chemotherapy-only approach even after adjustment for factors such as the number of first-line chemotherapy cycles and additional treatments.
The quality-of-life analysis according to the EORTC QLC-C30 questionnaire revealed sustained global health status/quality of life scores in the control group, while patients undergoing surgery reported deterioration for every outcome assessed. Quality of life by the EQ-5D questionnaire was markedly lower with pleurectomy/decortication than with chemotherapy alone throughout the study. Finally, in addition to the increased risk of death, more serious complications and poorer quality of life, the surgical approach was accompanied by higher costs of 20,102 $. The authors noted that relinquishing the concept of resectability in the context of mesothelioma would open access to effective systemic therapies currently licensed for disease that is defined as unresectable.
Figure: Reduction in overall survival with (extended) pleurectomy/decortication vs. chemotherapy alone
REFERENCES
- Kindler HD et al., Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36(13): 1343-1373
- NCCN Guidelines Pleural Mesothelioma, version 1.2023
- Popat S et al., Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines diagnosis, treatment and follow-up. Ann Oncol 2022; 33(2): 129-142
- Tsao AS et al., Current and future management of malignant mesothelioma: a consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol 2018; 13(11): 1655-1667
- Lim E et al., MARS 2: a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication of patients with malignant pleural mesothelioma. WCLC 2023, abstract PL03.10
© 2023 Springer-Verlag GmbH, Impressum
More posts
MARS 2: no benefit of decortication in early-stage malignant mesothelioma
MARS 2: no benefit of decortication in early-stage malignant mesothelioma Surgery in th
Preface – WCLC 2023
Preface – WCLC 2023 © private – Navneet Singh, MD DM FRCP FASCO, Professor of Pulmona