Esophageal cancer: taking immunotherapy one step further
In 2020, more than 604,000 new cases of esophageal cancer (EC) were diagnosed; EC was the sixth leading cause of cancer-related death worldwide [1]. Especially in Asia, the incidence of EC is high; for instance in China, its mortality rate reaches the fourth place of all deaths caused by cancer [2]. Esophageal squamous cell carcinoma (ESCC) accounted globally for approximately 85 % of all EC affected patients [3], with more than half of all ESCC cases worldwide are observed in China [4]. As most patients with ESCC are diagnosed in an advanced stage of the disease, their prognosis is poor, with an estimated 5-year survival rate of approximately 5 % [5]. Paclitaxel plus cisplatin or 5-FU plus cisplatin were the standard first-line therapy of advanced ESCC for almost two decades [6]. Considering that 1L chemotherapy leads to suboptimal overall survival (OS), alternative treatment options for this difficult-to-treat cancer with high related mortality (90 %) are scarce [1, 7].
In ESCC, PD-L1 overexpression (up to 62 %) is significantly associated with poor prognosis [8]. In previous clinical trials, the combination of an immune checkpoint inhibitor (CPI) and chemotherapy has demonstrated synergistic antitumoral activity [9]. However, only moderate improvements in terms of ORR and OS have been obtained so far with anti-PD-1 CPI versus chemotherapy as first-line treatment in patients with advanced ESCC [10], as well as for 2L therapy in patients with recurrent, locally advanced or metastatic ESCC who progressed on or after one prior line of systemic treatment [11, 12].
Immunotherapy benefit in ESCC
In the phase III ATTRACTION-3 trial, the anti-PD-1 CPI nivolumab used as monotherapy has proven to be superior to chemotherapy in terms of OS in patients with ESCC that was refractory or intolerant to previous chemotherapy [11]. Additionally, nivolumab plus ipilimumab already showed significant antitumoral efficacy across several tumor types [13].
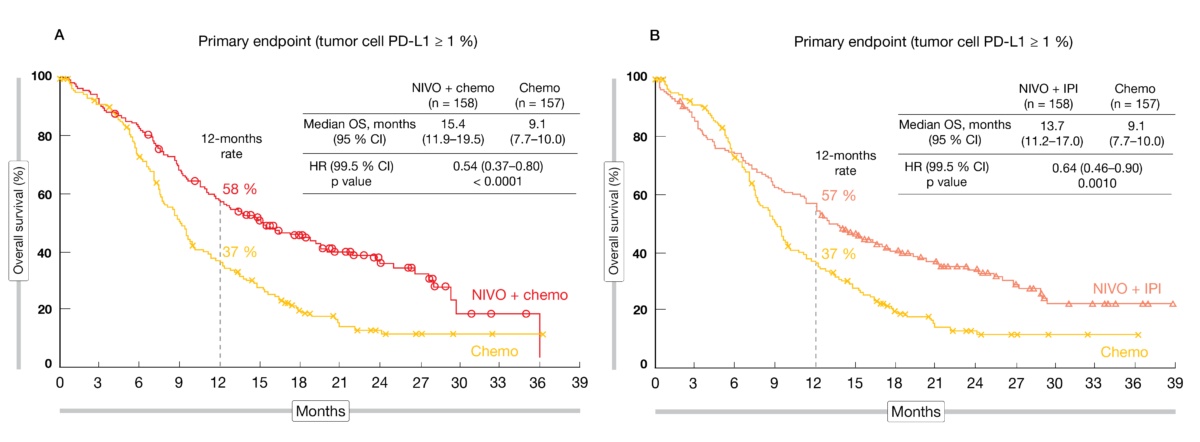
In the randomized, phase III CheckMate 648 study (NCT03143153), the efficacy and safety of nivolumab as front-line treatment were evaluated in patients with unresectable, advanced, recurrent, or metastatic ESCC following a 3-arm design (1:1:1) nivolumab plus chemotherapy (CT), nivolumab plus anti-CTLA-4 CPI ipilimumab, or chemotherapy alone. The treatment administered was either nivolumab (240 mg every other week [Q2W]) plus chemotherapy (fluorouracil + cisplatin Q4W), or nivolumab (3 mg/kg Q2W) plus ipilimumab (1 mg/kg Q6W), or chemotherapy alone until disease progression, discontinuation due to toxicity, withdrawal of consent or study end. OS and progression-fee survival (PFS) according to a blinded independent central review (BICR) in patients whose tumor cells expressed at least 1 % PD-L1 were the dual primary endpoints.
The primary analysis presented at ASCO 2021 included 970 enrolled patients [14]. After a minimum of 12.9 months follow-up, compared to chemotherapy alone, the regimen nivolumab plus chemotherapy showed a significant OS benefit compared to chemotherapy (15.4 vs 9.1 months; HR, 0.54; 99.5 % CI, 0.37-0.80; p < 0.0001) (Figure 1A) and a meaningful PFS advantage (6.9 vs 4.4 months; HR, 0.65; 98.5 % CI, 0.46-0.92; p<0.0023) in patients with tumor cells PD-L1 ≥ 1%. Similarly, median OS was significantly better in the nivolumab + ipilimumab arm compared to chemotherapy alone (13.7 vs 9.1 months; HR, 0.64; 99.5 % CI, 0.46-0.90; p < 0.001) (Figure 1B), but no PFS benefit was observed. Among patients with PD-L1 expressing tumors, the ORR reached 53 % in the nivolumab + chemotherapy arm versus 35 % in the nivolumab + ipilimumab arm versus 20 % with chemotherapy alone, while median DoR amounted to 8.4 versus 11.8 versus 5.7 months, respectively. Similar findings were observed regardless of PD-L1 expression.
In terms of safety, most common any-grade treatment-related adverse events (TRAEs) (≥ 10 %) included nausea, decreased appetite and stomatitis in the nivolumab plus chemotherapy arm (96 %), as well as in the chemotherapy (90 %) arm, whereas rash, pruritus and hypothyroidism were reported with nivolumab plus ipilimumab (80 %). Most selected side effects with potential immunologic etiology that require frequent monitoring/intervention experienced by study participants were grade 1 or 2; grades 3 and 4 TRAEs occurred in ≤ 6 % of patients across several organ categories. In comparison to previous studies with those CPIs, no new safety signals were detected.
The authors concluded that both nivolumab plus chemotherapy and the dual immunotherapy regimen are potential new 1L standards of care for patients with advanced ESCC, particularly for those with PD-L1 positive tumors.
Figure 1: Overall survival curves for nivolumab plus chemotherapy vs. chemotherapy alone (A) and nivolumab plus ipilimumab vs. chemotherapy (B).
RATIONALE 302: tislelizumab as 2L therapy of ESCC
Tislelizumab is an IgG4 monoclonal antibody against PD-1, which might avoid the development of resistance to anti-PD-1 therapy [15]. In early phase studies, the antitumoral activity of tislelizumab monotherapy was already shown in several solid tumors including ESCC [16]. Therefore, the authors hypothesized that tislelizumab might be an alternative treatment to the current chemotherapy standard.
RATIONALE 302 was a global, phase III trial (NCT03430843) that investigated the efficacy and safety of tislelizumab compared to chemotherapy in patients with histologically confirmed advanced/unresectable or metastatic ESCC, who progressed during or after a prior systemic therapy. Tislelizumab was administered at 200 mg intravenously (IV) every three weeks; the investigator-chosen therapy consisted of paclitaxel or docetaxel or irinotecan. The primary analysis of the data was presented at ASCO 2021 [17].
In total, 512 patients from 132 sites across 10 countries in Asia, Europe and North America, were randomized 1:1. The study met its primary endpoint as a significantly better median OS was observed with tislelizumab compared to the chemotherapy arm (8.6 vs 6.3 months; HR, 0.70; 95 % CI, 0.57-0.85; p = 0.0001). In patients with PD-L1 expression ≥ 10 % by vCPS, tislelizumab showed a clinically meaningful OS improvement – the key secondary endpoint – over chemotherapy with a 46 % reduction in the risk of death (10.3 vs 6.8 months; HR, 0.54; 95 % CI, 0.36-0.79; p = 0.0006) An OS benefit was consistently observed across all pre-defined subgroups. Compared to chemotherapy, a higher response rate was obtained with tislelizumab (ORR, 20.3 vs 9.8 %) and the responders showed a more durable DoR (7.1 vs 4.0 months; HR, 0.42; 95 % CI, 0.23-0.75).
Overall, 19 % of tislelizumab-treated patients experienced grade ≥ 3 TRAEs versus 56 % in the group who received chemotherapy. TRAEs leading to treatment discontinuation amounted to 7 % with tislelizumab and 14% with chemotherapy. No new safety signals were detected.
As tislelizumab showed a statistically significant and clinically meaningful improvement in OS compared to chemotherapy, as well as a better safety profile, the researchers concluded that it might be a potential new standard second-line therapy option for patients with advanced/unresectable or metastatic ESCC.
Synergy between immunotherapy and chemotherapy in ESCC
During a presentation at ASCO 2021 [18], the first author Rui-hua Xu spoke about a potential synergistic effect between immunotherapy and chemotherapy in patients with advanced or metastatic ESCC. The efficacy and safety of camrelizumab – an anti-PD-1 monoclonal antibody that already showed promising antitumoral activity in 2L ESCC (ESCORT trial) [19] – was thus evaluated in the phase III ESCORT-1st study (NCT03691090) as first-line therapy for advanced or metastatic ESCC patients. Study patients received either camrelizumab (200 mg) plus chemotherapy (paclitaxel and cisplatin for up to 6 cycles) or only the chemotherapy doublet IV Q3W. The co-primary endpoints were PFS assessed per IRC (independent review committee) and OS.
The first interim analysis presented the data of 596 eligible patients (median age of 62 years; mostly male patients) from 60 Chinese hospitals, who were randomized 1:1 to each arm. With a median follow-up of 10.8 months, a statistically significant median OS improvement was observed when camrelizumab was added to the dual chemotherapy regimen (15.3 vs 12.0 months; HR, 0.70; 95 % CI, 0.56-0.88; p = 0.001). Concerning the median IRC-PFS, camrelizumab plus chemotherapy reduced the risk or progression or death by 44 % compared to chemotherapy alone (6.9 vs 5.6 months; HR, 0.56; 95 % CI, 0.46-0.68; p < 0.001). Both OS and PFS benefits were observed in nearly all analyzed subgroups. In the immune-chemotherapy arm compared to the control arm, a higher response rate (ORR, 72.1 vs 62.1 %; CRs, 20 vs 11; PRs, 195 vs 174; SDs, 57 vs 80) and a longer DoR (7.0 vs 4.6 months) were observed.
A similar incidence of grade ≥ 3 TRAEs was observed in both patient groups; serious AEs occurred in 30.2 % of patients in the investigational arm and 23.2 % in the control arm. Camrelizumab plus chemotherapy showed a manageable safety profile and no new safety signals were identified.
Based on those findings, the author concluded that camrelizumab combined with paclitaxel plus cisplatin might be a new promising 1L therapy option for those patients; he also revealed that a new drug application dossier was already submitted to the China National Medical Products Administration for the approval of the combined immunochemotherapy in this setting.
Expanded analysis of CheckMate 649 in GC/GEJC/EAC
For patients with advanced or metastatic HER2-negative gastric cancer (GC) or gastroesophageal junction cancer (GEJC), 1L chemotherapy led to limited outcome, with a median OS of less than a year [20]. In the randomized, phase III CheckMate 649 study (NCT02872116), the efficacy and safety of first-line nivolumab plus chemotherapy versus chemotherapy alone was evaluated in advanced GC/GEJC/EAC (esophageal adenocarcinoma). In this trial, eligible patients with previously untreated, unresectable advanced or metastatic GC/GEJC/EAC – except those HER2-positive – were randomized 1:1:1 to receive either nivolumab (360 mg, Q3W or 240 mg, Q2W) plus chemotherapy (Xelox, Q3W or Folfox, Q2W) or nivolumab plus ipilimumab (Q3W x 4, then nivolumab 240 mg, Q2W) or only the chemotherapy regimen.
The first data of this trial were presented at ESMO 2020 [21]; the immunochemotherapy combination showed an OS superiority and a PFS benefit compared to chemotherapy alone. Based on those findings, nivolumab plus chemotherapy received FDA approval as 1L therapy for GC/GEJC/EAC in April 2021. At this year’s ASCO meeting, an expanded analysis of the CheckMate 649 was presented [22].
In this updated analysis, the OS (13.8 vs 11.6 months; HR, 0.80; 95 % CI, 0.68-0.94; p = 0.0002) and PFS (7.7 vs 6.9 months; HR, 0.77; 95 % CI, 0.68-0.87) benefits of the immunochemotherapy combination over chemotherapy in all randomized patients were confirmed. To note, OS advantage was observed across multiple prespecified subgroups. A higher response was obtained in the investigational arm compared to the control arm (ORR, 58 vs 46 %; CRs, 10 vs 6; PRs, 48 vs 40; SDs, 28 vs 33); this ORR benefit was observed regardless of the PD-L1 expression status and was more durable (DoR, 8.5 vs 6.9 months, respectively).
In total, TRAEs grade 3-4 were experienced by 59 % of patients in the nivolumab plus chemotherapy group and in 44 % of those in the chemotherapy group. Concerning the HR-QoL, patients in the investigational arm showed a decreased risk of symptom deterioration on treatment compared to the patients in the control group (HR, 0.77; 95 % CI, 0.63-0.95; p = 0.0129).
These updated data further support this immunochemotherapy as first-line standard treatment in patients with advanced HER2-negative GC/GEJC/EAC.
Pembrolizumab in neoadjuvant setting in EAC patients
In patients with resectable EC or GEJC, neoadjuvant chemoradiotherapy (CRT) has been shown to improve survival [23]. Recently, adjuvant anti-PD-1 nivolumab treatment following neoadjuvant CRT has been demonstrated to be beneficial in resected EC/GEJC patients with residual disease (CheckMate 577 trial) [24]. In the Keynote-590 study, the efficacy of anti-PD-1 pembrolizumab plus chemotherapy has been proven as efficient and safe 1L treatment of EC/GEJC patients [25]. Therefore, one might hypothesize that the addition of pembrolizumab to CRT in the neoadjuvant setting may further improve the outcome of locally advanced EAC patients.
In a randomized, phase II study, patients with T2-4 or N+ non-metastatic, resectable EAC or GEJC were randomized 1:1 to receive either full-dose paclitaxel (T)/ carboplatin (C) or T/C + pembrolizumab as preoperative therapy (NCT02998268). All study patients got neoadjuvant CRT, consisting in weekly dual chemotherapy with 41.4 Gy in 23 radiotherapy fractions; in the investigational arm, pembrolizumab was additionally administered every 3rd week. Following resection, all patients received pembrolizumab for one year. The rate of major pathologic response (MPR, defined as pathologic CR or near CR [< 10 % residual disease]) was primary assessed [26].
Among the 39 enrolled patients (15 with EC or type I GEJC, 24 with type II or III GEJC), 79.5 % were male and the median age was 68 years. MPR rate was 48.7 % (95 % CI, 33.0-64.4) at the time of the analysis. Overall, 1-year OS rate were 77.5 % (95 % CI, 56.4-89.3), with a 1-year OS rate of 93.8 % (95 % CI, 63.2-99.1) in patients with MPR and 62.5 % (95 % CI, 31.5-82.6) in those without MPR. Similarly, 1-year DFS was 60.4 % (95 % CI, 39.3-76.2), with 100.0 % achieved by patients with MPR and 23.5 % without MPR (95 % CI, 5.8-47.9; p = 0.001). Interestingly, patients with EC showed a significantly higher MPR rate than those with GEJC (73.3 vs 33.3 %). This might be explained by a different tumor immune microenvironment in those tumor entities; indeed, EAC or GEJC type I tumors presented a greater infiltration of activated dendritic cells (p = 0.12), whereas GEJ tumors showed a significantly higher infiltration of activated B cells (p = 0.02).
Pembrolizumab plus CRT was well tolerated. Post-surgery, typical post-operative AEs – including wound dehiscence, infections, atrial fibrillation, and cardiac toxicities – were reported, while the most common toxicities of interest grade 3-4 observed were elevated liver enzymes (13.9 %), pneumonitis (11.1 %), elevated blood sugar (8.3 %) and adrenal insufficiency (2.8 %).
In resectable EC or GEJC patients, the combination of pembrolizumab plus CRT as neoadjuvant therapy, followed by pembrolizumab adjuvant treatment, was safe and more efficient than CRT alone in terms of MPR, DFS and OS. Two follow-up clinical studies are currently investigating the benefit of the addition of pembrolizumab (Keynote-975) or nivolumab (EA2174) to CRT in the neoadjuvant setting.
New doublet CPI as combined therapy for ESCC
KN046 is the first dual CPI which has been designed to block both PD-1/PD-L1 and CTLA-4 pathways simultaneously. Its efficacy and safety as monotherapy or in combination with chemotherapy is currently under evaluation in a phase II study in China. Patients with histologically or cytologically confirmed unresectable, locally advanced, recurrent, or metastatic ESCC are included in the study cohort (NCT03925870).
The preliminary results of the cohort 3 (1L therapy) were recently presented at ASCO 2021 [27]. Eligible patients are receiving KN046 (5 mg/kg) plus paclitaxel (135-175 mg/m2) and cisplatin (75 mg/m2) intravenously Q3W for four to six cycles; additionally, in patients without progressive disease, a maintenance therapy with KN046 monotherapy (Q2W) is administered until progression or unacceptable toxicity. Investigator-assessed ORR per RECIST v1.1 is the primary endpoint.
At the time of analysis, among 15 male patients (median age, 63 years; 80 % stage IV) already enrolled, twelve, who had at least one tumor assessment, entered the evaluable analysis set (EAS). The ORR was 58.3 % (95 % CI, 21.1-78.9), while the DCR amounted to 91.7 % (95 % CI, 61.5-99.8; 4 PRs, 3 unconfirmed PRs and 4 SDs). One patient had a progressive disease (PD).
The incidence of KN046-associated AEs was 80.0 %, 13.3 % of them being grade ≥ 3 TRAEs. Immune-related AEs (irAEs) of any grade were observed in 53.3 % of patients; most common grade ≥ 3 irAEs experienced by 13.3 % of patients were nausea (n = 1, 6.7 %) and rash (n = 1, 6.7 %).
The study group concluded that KN046 combined to the dual chemotherapy paclitaxel plus cisplatin is an efficient and safe first-line treatment, and therefore a potential new therapeutic option for patients with advanced ESCC.
AdvanTIG-203: dual targeting in ESCC
Because of resistance mechanism, a durable outcome remains an unmet need in patients with recurrent, locally advanced, or metastatic ESCC who progressed on or after one prior line of systemic treatment. TIGIT is a co-inhibitory immune checkpoint receptor expressed on immune cells in multiple solid tumors [28]. Ociperlimab is a humanized, monoclonal antibody targeting TIGIT with a highly specific binding activity; thereby, it activates the antitumoral immune response through T-cells and natural killer cells [29]. The anti-PD-1 tislelizumab has been designed to have a minimal binding to Fcy receptor on macrophages to abolish the antibody-dependent phagocytosis, a mechanism involved in resistance to anti-PD-1 treatment [15]. The OS superiority of tislelizumab over chemotherapy in the 2L treatment of patients with advanced or metastatic ESCC was presented at this year’s ASCO meeting [17]. Moreover, a dual targeting of tumors with an anti-TIGIT and anti-PD-1 has been already shown to result in a synergistic immune cell activation in early phase studies [30].
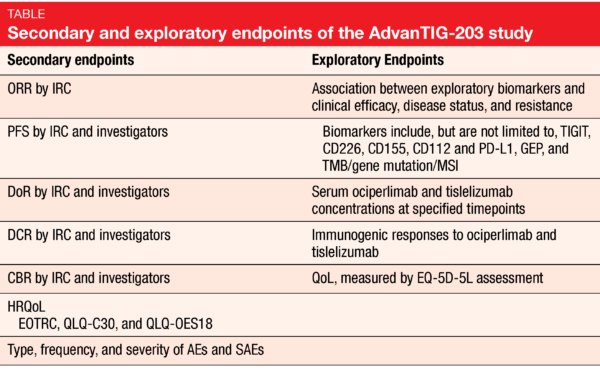
At ASCO 2021, the design of a new randomized, double-blind, phase II study named AdvanTIG-203 (NCT04732494) – which aims to evaluate the efficacy and safety of ociperlimab plus tislelizumab – was presented. This trial is currently enrolling patients with histologically confirmed unresectable, locally advanced, recurrent or metastatic ESCC, who are progressive following first-line systemic therapy and whose tumors express PD-L1 (CPS ≥ 10) [31]. This trial intends to randomize 140 patients in each study arms: in arm A, patients receive IV Q3W ociperlimab (900 mg) plus tislelizumab (200 mg), while patients in arm B receive tislelizumab plus placebo. The OS and ORR are the co-primary study endpoints; the multiple secondary and exploratory endpoints are described in the Table. Concerning the safety assessments, AEs, serious AEs (SAEs) and irAEs will be reported.
MATTERHORN: neoadjuvant-adjuvant durvalumab in GC/GEJC
In 2020, gastric cancer (GC) was the sixth more common cause of cancer worldwide and responsible of more than 760, 000 deaths (mortality rate of 71 %) [1]. In Western countries, neoadjuvant-adjuvant FLOT (5-fluorouracil + leucovorin + oxaliplatin + docetaxel) chemotherapy is the standard of care of resectable GC/GEJC [32]; in East Asian countries, surgery – followed by adjuvant chemotherapy and eventually preceded by perioperative chemotherapy – is the current treatment in this setting [33]. Despite improved OS outcome thanks to new treatment advances, patients with GC/GEJC have a poor prognosis, mostly due to a high recurrence rate [34]. Previous evidence suggested that the combination of an anti-PD-1 CPI and cytotoxic chemotherapy as neoadjuvant-adjuvant treatment may result in increased efficacy [35].
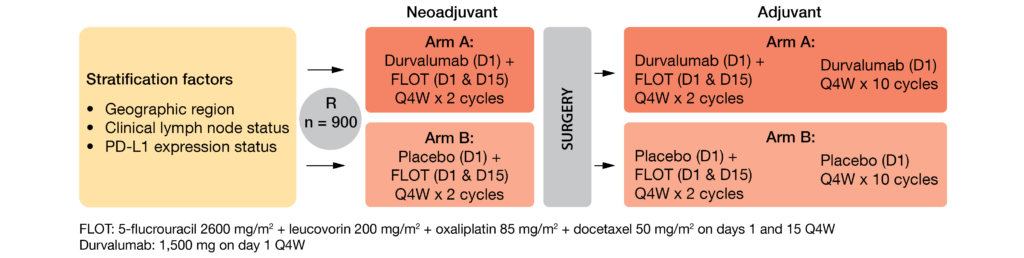
The randomized, double-blind, ongoing, global, multicenter, phase III MATTERHORN study (NCT04592913) evaluates FLOT chemotherapy plus neoadjuvant-adjuvant anti-PD-1 durvalumab or placebo – followed respectively by adjuvant durvalumab or placebo – in patients with histologically confirmed (stage II or higher) resectable GC/GEJC [36]. This trial is open for enrolment and intend to enroll 900 anticancer therapy-naïve patients equally either in the investigational group (arm A) or in the control group (arm B) following the design described in Figure 2. The key inclusion criteria include a complete surgical resection of the primary tumor, as well as a performance status ≤ 1. Patients who received any prior immune-mediated therapy, as well as those who have peritoneal dissemination or distant metastasis, (adeno)squamous cell carcinoma or gastrointestinal stromal tumor, will be excluded. The primary endpoint is event-free survival (EFS) assessed by BICR and/or pathology testing; the secondary endpoints enclose OS, pathological complete response (pCR) rate, safety, and tolerability profile.
Figure 2: MATTERHORN study design
KEYNOTE-811 study
In this ongoing, global, randomized, double-blind, placebo-controlled, phase III study (NCT03615326), the addition of the anti-PD-1 pembrolizumab (200 mg IV Q3W) to the standard-of-care (SOC) 1L therapy (trastuzumab plus chemotherapy) was evaluated in unresectable or metastatic HER2+ G/GEJ cancer [37]. From the first 264 enrolled patients, the confirmed ORR was higher with the investigational combination compared to SOC (74.4 vs 51.9 %; 95 % CI, 11.2-33.7; p = 0.00006), with a CR rate of 11.3 vs 3.1 %. The addition of pembrolizumab to SOC led to a longer median DoR (10.6 vs 9.5 months with SOC). Grade 3-5 AEs were experienced by 57.1 % of patients in pembrolizumab + SOC arm versus 57.4 % with SOC; discontinuation rate was 24.4 vs 25.9 %, respectively.
Based on these results showing a durable response and a manageable safety, the triple regimen was approved by the FDA for this patient population in May 2021.
REFERENCES
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- International Agency for Research on Cancer, WHO, Globocan. China. 2020; Available from: https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf.
- Arnold M et al., Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020; 69(9): 1564-1571.
- Hou H et al., Survival of Esophageal Cancer in China: A Pooled Analysis on Hospital-Based Studies From 2000 to 2018. Front Oncol 2019; 9: 548.
- Howlader N SEER Cancer Statistics Review (CSR) 1975-2016. 2020; Available from: https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_08_esophagus.pdf.
- Lordick F et al., Oesophageal cancer: ESMO clinical practice guidelines. Ann Oncol 2016; 27(suppl 5): v50-v57.
- Moehler M et al., Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol 2020; 31(2): 228-235.
- Wang Q et al., PD-L1 Expression On tumor Cells Was Associated With Unfavorable Prognosis In Esophageal Squamous Cell Carcinoma. J Cancer 2018; 9(12): 2224-2231.
- Baba Y et al., Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci 2020; 111(9): 3132-3141.
- Kato K et al., Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol 2020; 31(suppl 4): S1142-S1215.
- Kato K et al., Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(11): 1506-1517.
- Vivaldi C et al., Immune Checkpoint Inhibitors in Esophageal Cancers: are we Finally Finding the Right Path in the Mist? Int J Mol Sci 2020; 21(5).
- Klein O et al., Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res 2020; 26(17): 4454-4459.
- Chau I et al., Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol 2021; 39(suppl 15; abstr LBA4001).
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Shen L et al., Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8(1): e000437.
- Shen L et al., RATIONALE 302: Randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol 2021; 39(suppl 15; abstr 4012).
- Xu R et al., ESCORT-1st: A randomized, double-blind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy versus chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC). J Clin Oncol 2021; 39(suppl 15; abstr 4000).
- Huang J et al., Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020; 21(6): 832-842.
- Fuchs CS et al., Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(3): 420-435.
- Moehler M Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 2020; 31 (suppl 4): S1142-S1215.
- Moehler M et al., First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): Expanded efficacy and safety data from CheckMate 649. J Clin Oncol 2021; 39(suppl 15; abstr 4002).
- van Hagen P et al., Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366(22): 2074-84.
- Kelly RJ et al., Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021; 384(13): 1191-1203.
- Kato K et al., KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol 2019; 15(10): 1057-1066.
- Shah M et al., Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC). J Clin Oncol 2021; 39(suppl 15; abstr 4005).
- Xu J et al., Efficacy and safety of KN046 plus paclitaxel/cisplatin as first-line treatment for unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma (ESCC). J Clin Oncol 2021; 39(suppl 15; abstr 4062).
- Harjunpää H et al., TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020; 200(2): 108-119.
- Frentzas S et al., ADVANTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. AACR 2021, Poster 2583.
- Rodriguez-Abreu D et al., Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol 2020; 38(suppl 15).
- Xu, R et al., AdvanTIG-203: A randomized phase 2 study comparing anti-TIGIT ociperlimabplus tislelizumab versus tislelizumab plus placebo as second-line treatment inpatients with advanced or recurrent esophageal squamous cell carcinoma(ESCC) expressing programmed death-ligand 1 (PD-L1). J Clin Oncol 2021; 39 (suppl 15; abstr TPS4150).
- Al-Batran, SE et al., Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393(10184): 1948-1957.
- Tokunaga, M et al., Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today 2020; 50(1): 30-37.
- Sexton, RE et al., Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 2020; 39(4): 1179-1203.
- Yu, WD et al., Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett 2019; 452: 66-70.
- Janjigian, Y et al., MATTERHORN: Efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer—A randomized, double-blind, placebo-controlled, phase 3 study. J Clin Oncol 2021; 39(suppl 15; abstr TPS4151).
- Janjigian Y et al., Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol 2021; 39(suppl 15; abstr 4013).
© 2021 Springer-Verlag GmbH, Impressum
More posts
Early insights for CPI combinations in solid tumors
Early insights for CPI combinations in solid tumors Ezabenlimab combined with a
Anti-PD-1 compounds targeting MSI-H/dMMR tumors
Anti-PD-1 compounds targeting MSI-H/dMMR tumors Keynote-158: an update of pembr
PARP- and anti-PD-1-based strategies in breast and cervical cancer
PARP- and anti-PD-1-based strategies in breast and cervical cancer Talazoparib
Checkpoint inhibition: predictors, resistance and immunogenomic features
Checkpoint inhibition: predictors, resistance and immunogenomic features Predic
Novel approaches in gastric cancer
Novel approaches in gastric cancer LEAP-005: lenvatinib plus pembrolizumab in g
Preface ASCO Solid Tumor 2021
Preface ASCO Solid Tumor 2021 Ian Chau, MD, Department of Medicine, The Royal M