Expansion of clinical trial enrollment criteria: what would we gain?
Broadened vs. traditional: retrospective analysis
In 2017, the American Society of Clinical Oncology and the non-profit organization Friends of Cancer Research noted in their joint statement that trial enrollment criteria should strive for inclusiveness to make trial populations more representative and to maximize generalizability of findings [1]. Also, this would enable more patients to participate and accelerate accrual, resulting in expedited availability of new therapies. Harvey et al. conducted a retrospective study using real-world data obtained between January 2011 and December 2018 to demonstrate the impact of broadened versus traditional criteria on the eligibility of patients with advanced NSCLC [2]. Based on the ASCO CancerLinQ Discovery (CLQD) deidentified electronic health record, patients who received treatment after a diagnosis of advanced NSCLC were identified. Outcome measures related to the number and characteristics of patients eligible by traditional vs. broadened criteria. Specifically, three domains of criteria were evaluated, i. e., prior and concurrent cancers, brain metastases, and kidney function. Patients with a cancer history, brain metastases and creatinine clearance ≤ 60 mL/min are usually excluded from clinical studies. According to the broadened criteria, all cases with another primary cancer diagnosis were included, as well as all patients with brain metastases irrespective of treatment status and clinical stability, and those with creatinine clearance ≥ 30 mL/min.
Doubling of eligible patients
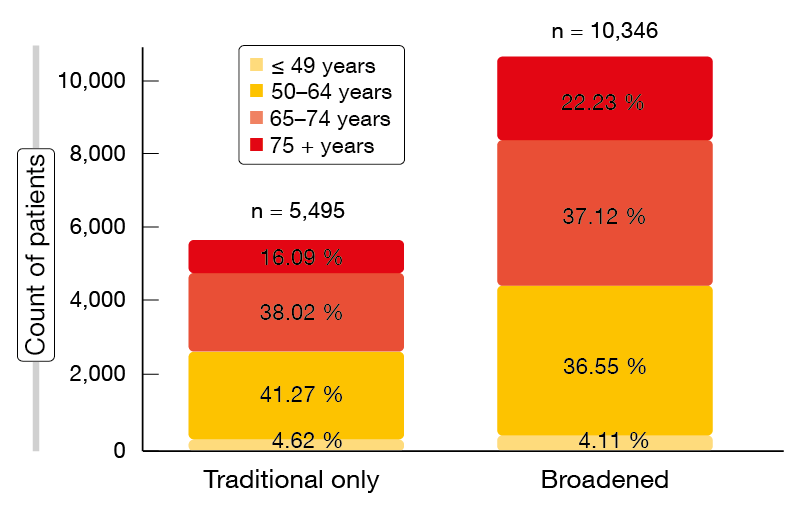
Within the total group of 10,500 patients, according to the traditional criteria, the proportions of patients excluded due to prior/concurrent cancers, brain metastases, and creatinine clearance ≤ 60 mL/min were 21.5 %, 21.2 %, and 14.4 %, respectively. Overall, 47.7 % of these patients would not have been able to participate in clinical trials. The broadened criteria, on the other hand, only prompted exclusion of 1.5 % based on the creatinine clearance cut-off. Thus, the traditional and broadened cohorts comprised 5,495 and 10,346 patients, respectively, with the broadened cohort containing a comparably higher percentage of patients aged > 75 years (22.23 % vs. 16.09 %; Figure). This analysis shows that the use of expanded criteria would enable almost twice as many patients with advanced NSCLC to consider trial participation. Moreover, these criteria are likely to result in trial participants being more reflective of a broader patient population. The authors noted that narrower criteria should only be used based on a compelling scientific rationale. Additional recommendations by ASCO and Friends of Cancer Research are in progress.
Figure: Distribution of age groups in the traditional and broadened cohorts
REFERENCES
- Kim ES et al., Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017; 35(33): 3737-3744
- Harvey RD et al., Impact of broadening clinical trial eligibility criteria for advanced non-small cell lung cancer patients: real-world analysis. J Clin Oncol 37, 2019 (suppl; abstr LBA108)
More posts
Small-cell tumors: improvements in the second-line setting
Small-cell tumors: improvements in the second-line setting Lurbinectedin monoth
Rare mutations: taking treatment one step further
Rare mutations: taking treatment one step further GEOMETRY mono-1: capmatinib i
Interview: Blood-based testing in ALK-positive disease
Blood-based testing in ALK-positive disease Interview: Rafał Dziadziuszko, MD, PhD
Trial updates and new biomarkers in the field of immunotherapy
Trial updates and new biomarkers in the field of immunotherapy Long-term findin
Novel first-line options and other insights in EGFR-mutant lung cancer
Novel first-line options and other insights in EGFR-mutant lung cancer RELAY: a
Early-stage NSCLC: promising (neo)adjuvant approaches
Early-stage NSCLC: promising (neo)adjuvant approaches The NEOSTAR trial Effecti