Rare mutations: taking treatment one step further
GEOMETRY mono-1: capmatinib in MET-dysregulated NSCLC
MET exon 14 skipping mutations (METex14) have been reported in 3 % to 4 % of NSCLC patients [1-3]. They confer poor prognosis and poor responses to standard therapies including immunotherapy [4-8]. Moreover, patients with MET alterations are generally older, which implies that tolerable strategies are called for. Capmatinib has been developed as a highly selective, potent MET inhibitor with in vitro and in vivo activity against preclinical cancer models harboring MET activation [9].
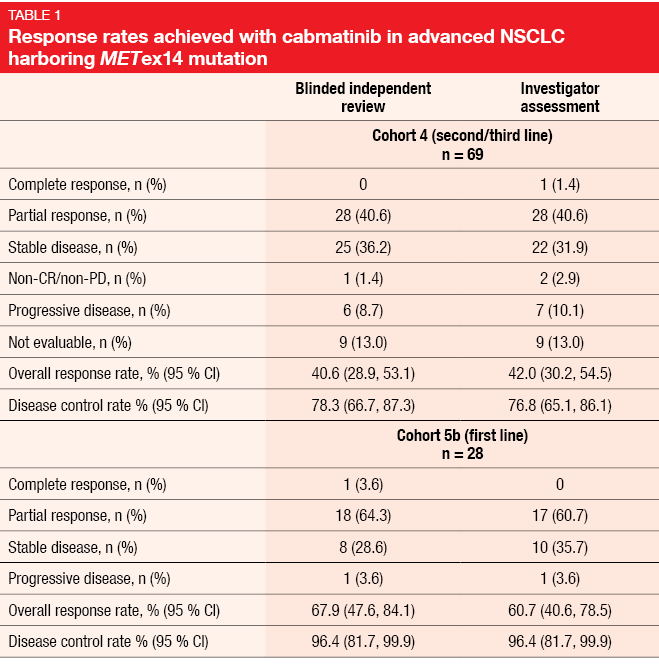
The multicohort, multicenter, phase II GEOMETRY mono-1 trial investigated capmatinib 400 mg twice daily in patients with stage IIIB/IV NSCLC and METex14. Preliminary efficacy data presented at ESMO 2018 have shown deep responses irrespective of the line of treatment, as well as CNS activity [10]. At the ASCO 2019 Congress, Wolf et al. reported the primary efficacy analysis and other analyses for Cohorts 4 and 5b [11]. Cohort 4 evaluated capmatinib in the second and third line (n = 69), while Cohort 5b included treatment-naïve patients (n = 28). In the pretreated cohort, 74 % had received one treatment line; here, platinum-based chemotherapy had been administered in 88.4 %. Most of the patients in both cohorts showed concurrent MET amplification. The two cohorts were analyzed separately and had independent, prospectively designed statistical hypotheses. ORR according to the blinded independent review committee (BIRC) constituted the primary endpoint.
Extra- and intracranial effects
In Cohort 4, ORR by BIRC amounted to 40.6 %, while DCR, as the key secondary endpoint, was 78.3 % (Table 1). For Cohort 5b, ORR and DCR were 67.9 % and 96.4 %, respectively. Rapid, deep and durable responses occurred across both cohorts. Median duration of response was 9.72 and 11.14 months in Cohorts 4 and 5b, respectively. At 12 months, 25.8 % and 49.7 % of patients, respectively, remained progression-free; median PFS was 5.42 and 9.69 months. All of these outcomes were consistent between BIRC and investigator assessment. The neuro-radiologist review confirmed activity of the capmatinib treatment against brain metastases. Seven of 13 evaluable patients who had CNS lesions at baseline achieved intracranial responses, with four patients even experiencing complete resolution of all metastases. Twelve patients obtained intracranial disease control. Intracranial responses were demonstrated to develop as rapidly as those observed outside of the CNS.
Furthermore, the investigators found that deep and lasting responses occurred independently of the type of MET mutation leading to METex14 or co-occurrence of MET amplification. Both next generation sequencing and reverse transcriptase polymerase chain reaction demonstrated high sensitivity for the detection of METex14 in tumor tissue, with a concordance rate of 99 %. As is the case for other molecular drivers, tumor mutational burden was low in these patients (median, < 6 mut/MB in tumor tissue) compared to those with wild-type NSCLC, and similar across treatment lines.
The safety analysis dataset represents the largest dataset of MET-dysregulated NSCLC patients up to now (n = 334). Capmatinib showed high tolerability, with few grade-3/4 events. Peripheral edema, nausea, and increased creatinine levels were the most frequently reported AEs. Dose adjustments and treatment discontinuation due to treatment-related AEs became necessary in 21.9 % and 11.1 %, respectively. In their summary, the authors pointed out that the favorable ORR in the treatment-naïve cohort highlights the importance of early molecular testing. Capmatinib appears to be a new treatment option in the rare but challenging population of patients with advanced NSCLC and MET dysregulation.
Tepotinib: the VISION study
Another highly selective, potent MET inhibitor is tepotinib, which is being evaluated in the single-arm phase II VISION trial in patients with stage IIIB/IV NSCLC of all histologies and MET alterations according to tissue or liquid biopsy. In Cohort A, patients with METex14 skipping mutations are receiving tepotinib 500 mg/d until progression. Tepotinib is used in the first-, second- and third-line settings. Paik reported interim findings including ORR assessed by independent review (i.e., the primary endpoint) and select secondary outcomes for Cohort A [12]. Eighty-seven patients had been treated at the time of the analysis.
Tepotinib elicited ORRs of 50.0 % and 45.1 % by independent review according to liquid biopsy and tissue biopsy, respectively. Reponses lasted for 12.4 and 15.7 months, respectively. Disease control was achieved in 66.7 % and 72.5 %, respectively. The treatment activity was consistent across treatment lines. This also held true for tumor shrinkage; 92 % of patients according to both independent review and investigator read experienced tumor shrinkage in the first- and second-line settings. In the third line and beyond, evidence of tumor shrinkage was found in ≥ 75 % of cases. Responses occurred early on and were durable across treatment lines. Median duration of response exceeded one year in all analysis subsets and was 14.3 months overall. Patients who showed brain metastases at baseline benefitted equally from treatment. Median PFS was 9.5 and 10.8 months in the total cohort according to liquid biopsy and tissue biopsy, respectively.
The trial demonstrated a favorable safety profile, with peripheral edema, nausea, and diarrhea reported as the most common AEs. No grade 4 or 5 treatment-related AEs occurred. The investigators concluded that tepotinib shows promising and durable clinical activity in patients with METex14 mutations. The VISION study is ongoing; results for Cohort B, which includes patients with MET amplification in the absence of METex14 skipping mutations, will be presented in the future.
BLU-667 for RET-positive disease
RET alterations are found in approximately 1 % to 2 % of NSCLC cases [13, 14]. There is an unmet medical need in these patients, as no significant benefits from existing strategies such as chemotherapy, immunotherapy or multikinase inhibitor treatment have been observed for them [15-17]. No selective RET inhibitors have been approved to date.
The investigational agent BLU-667, which potently and selectively inhibits RET alterations and RET resistance mutants [18, 19], might be on the verge of filling this gap. Gainor et al. presented findings from the phase I ARROW study [20]. The dose-escalation part of this trial identified 400 mg/d as the ideal dose. Part 2 is currently enrolling patients into seven expansion cohorts with various RET-altered advanced solid tumors. Asymptomatic brain metastases are allowed. Two of the cohorts contain NSCLC patients, with one cohort being platinum-naïve and the other being platinum-pretreated. The latter had received a median of two prior lines of therapy. Forty percent in the overall NSCLC population showed CNS lesions. Known RET fusion partners were mainly KIF5B (66 %) and CCDC6 (13 %). ORR and safety constitute the primary objectives of the ARROW study.
Clinical benefits and RET clearance
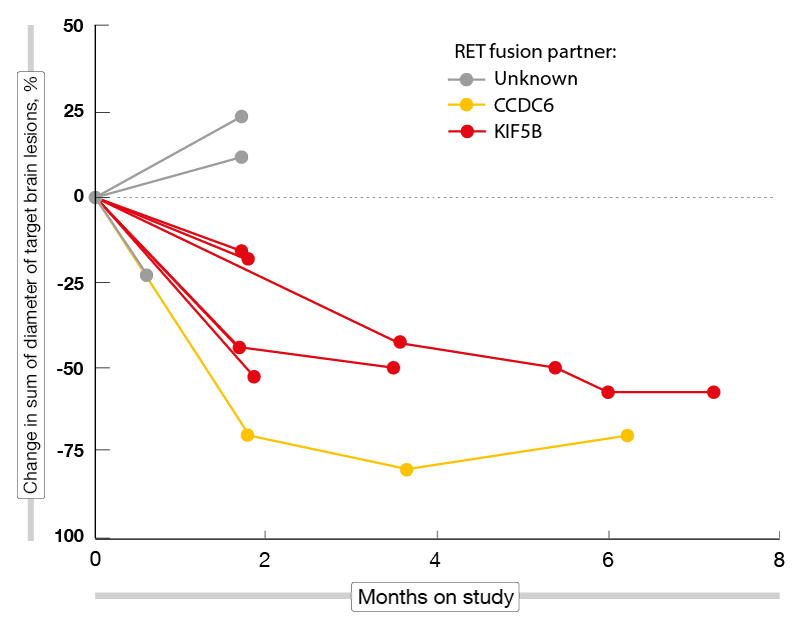
According to the preliminary efficacy analysis conducted in 48 NSCLC patients 35 of whom were pretreated, BLU-667 demonstrated broad and durable anti-tumor activity, with ORRs of 58 % and 60 % in the total cohort and the pretreated group, respectively. DCRs were 96 % and 100 % for these two populations. Most responses emerged already at the time of the first follow-up imaging assessment. At data cutoff, 82 % of responding patients remained on treatment, and the median duration of response had not been reached yet. Including the dose-escalation phase, the patients have been on treatment for up to 24 months. BLU-667 retained activity irrespective of prior immune checkpoint inhibitor treatment, RET fusion genotypes, and CNS involvement. Seven out of nine patients who had measurable untreated brain metastases at baseline achieved shrinkage of these lesions (Figure). No patient treated with a starting dose of 400 mg/d experienced progression due to new CNS involvement. Eighteen of 20 patients with detectable RET fusion ctDNA at baseline showed complete clearance within the first treatment cycle.
The safety analysis comprised 120 patients, 91 of whom were platinum-pretreated. BLU-667 was well tolerated, with toxicities generally being low-grade, reversible and consistent with the drug’s selectivity profile. The most common AEs included constipation, neutropenia, transaminase elevations, fatigue, and hypertension. Among grade ≥ 3 AEs, neutropenia and hypertension prevailed in 13 % each. In total, 7 % of patients discontinued BLU-667 due to treatment-related toxicity. As the authors noted, these data support the expansion of the ARROW trial in treatment-naïve NSCLC patients.
Figure: Shrinkage of brain metastases in RET-positive lung cancer patients receiving BLU-667
Convincing larotrectinib activity
Rearrangements involving the NTRK genes have been identified across a broad range of malignancies, with an estimated frequency of 1 % in all solid tumors [21]. The first-in-class, highly selective TRK inhibitor larotrectinib demonstrated robust efficacy in an integrated expanded dataset of 109 patients regardless of tumor type or age [22]. Hong et al. evaluated the efficacy and safety of larotrectinib 100 mg twice daily in 83 adult patients with locally advanced or metastatic solid tumors treated in three clinical trials (adult phase I, NCT02122913; SCOUT, NCT02637687; NAVIGATE, NCT02576431) [23]. Patients with a total of 12 tumor types had been included in these studies; 13 % of them had been diagnosed with lung cancer.
Larotrectinib was shown to induce strong and durable responses in the entire population. ORR by independent review committee was 68 %. CR, PR and SD were observed in 17 %, 51 %, and 15 %, respectively. Responses occurred irrespective of tumor type. At a median follow-up of 17.5 months, median duration of response had not been reached yet for patients with confirmed responses. Seventy-nine percent of responders were estimated to be in response longer than 12 months. Median PFS was 25.8 months, and median OS had not been reached.
Larotrectinib was well tolerated, with the majority of AEs being graded as 1 or 2. The most common AEs included fatigue (40 %), dizziness (36 %), and nausea (29 %). Overall, these data provide strong evidence in support of testing for TRK fusions in adult patients with advanced solid tumors regardless of the site of the primary tumor.
Characteristics of NRG1-positive lung cancer
NRG1 fusions are found in approximately 1.7 % of patients with adenocarcinoma of the lung [24]. These fusions activate HER3/HER2 signaling, supporting the therapeutic use of HER3 and/or HER2 inhibitors. However, characterization of clinicopathological and molecular features of this disease is lacking, as well as evidence on the efficacy of systemic treatments in a large cohort of patients with NRG1-positive NSCLC. Duruisseaux et al. therefore launched a registry involving a global, multicenter network of thoracic oncologists from 17 institutions in eight countries [25]. These identified a total of 117 patients who had confirmed NRG1-fusion–positive NSCLC. Clinicopathological/molecular features and clinical outcomes were collected retrospectively.
The cohort contained a high proportion of women (54.7 %) and never smokers (43.6 %). A median of 40 pack years was reported for smokers. The tumors mainly showed adenocarcinoma histology (94.9 %), with the mucinous subtype dominating (71 %). In terms of genetic characteristics, NRG1 fusions had upstream partner genes in 58.9 % of cases; here, CD74 and SCLA3A2 were most common. NRG1 fusions were mainly identified using RNA-based assays. In patients who had metastatic disease, the lung was the most common organ site of dissemination. Stage-IV NRG1-positive NSCLCs showed a remarkably good prognosis, with median OS of 4.83 years. For stages I and III, median OS had not been reached yet, and for stage II, this was 4.4 years.
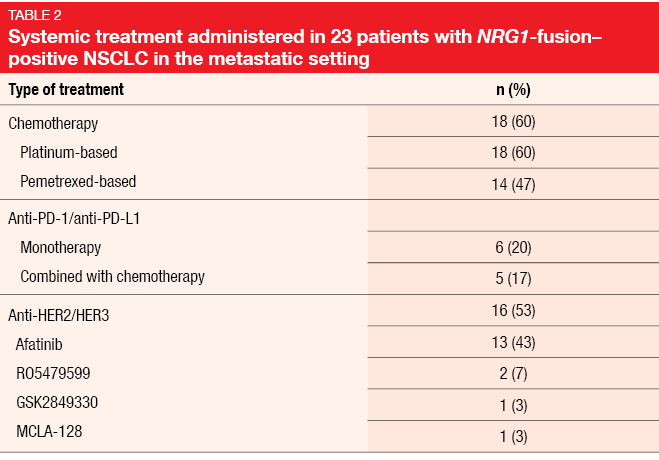
Data on the efficacy of systemic therapies were available for 23 patients. Platinum-based chemotherapy was administered in 18 cases (Table 2). Here, two patients obtained PR (11 %), and SD occurred in nine individuals (50 %). Among patients treated with afatinib as a single agent or in combination (n = 13, with efficacy data available for 12), one achieved CR (8 %), while three developed PR (25 %) and two SD (17 %). Two patients experienced responses lasting for more than one year. Median PFS with afatinib was 2.0 months, while median OS had not been reached yet. However, OS from the diagnosis of the metastatic stage did not differ across patients with and without afatinib treatment. For single-agent anti-PD-1/L1 therapy, no responses were observed; this also applied to chemoimmunotherapy.
The authors concluded that afatinib treatment might not change the natural history of the metastatic disease, although long-lasting responses occurred with this treatment in a few patients. Novel targeted therapeutic approaches are called for. RNA-based assays might be the test method of choice for the identification of NRG1 fusions.
REFERENCES
- Ma PC, MET receptor juxtamembrane exon 14 alternative spliced variant: novel cancer genomic predictive biomarker. Cancer Discov 2015; 5(8): 802-805
- Reungwetwattana T et al., The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer 2017; 103: 27-37
- Tong JH et al., MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016; 22(12): 3048-3056
- Dimou A et al., MET gene copy number predicts worse overall survival in patients with non-small cell lung cancer (NSCLC); a systematic review and meta-analysis. PLoS One 2014; 9(9): e107677
- Guo B et al., Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One 2014; 9(6): e99399
- Sabari JK et al., PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol 2018; 29(10): 2085-2091
- Baba K et al., Efficacy of pembrolizumab for patients with both high PD-L1 expression and a MET exon 14 skipping mutation: A case report. Thorac Cancer 2019; 10(2): 369-372
- Reis H et al., MET expression in advanced non-small-cell lung cancer: Effect on clinical outcomes of chemotherapy, targeted therapy, and immunotherapy. Clin Lung Cancer 2018; 19(4): e441-e163
- Baltschukat S et al., Capmatinib (INC280) is active against models of non-small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin Cancer Res 2019; 25(10): 3164-3175
- Wolf J et al., Results of the GEOMETRY mono-1 phase II for evaluation of the MET inhibitor capmatinib (INC280) in patients with METΔex14 mutated advanced non-small cell lung cancer. ESMO 2018, abstract LBA52
- Wolf J et al., Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol 37, 2019 (suppl; abstr 9004)
- Paik PK et al., Phase II study of tepotinib in NSCLC patients with METex14 mutations. J Clin Oncol 37, 2019 (suppl; abstr 9005)
- Lipson D et al., Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012; 18(3): 382-384
- Takeuchi K et al., RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012; 18(3): 378-381
- Mazieres J et al., Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). J Clin Oncol 36, 2018 (suppl; abstr 9010)
- Drilon A, Targeted therapy outcomes in RET-rearranged lung cancers: drug or driver? Lancet Respir Med 2017; 5(1): 5-6
- Yoh K et al., Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017; 5(1): 42-50
- Subbiah V et al., Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov 2018; 8(7): 836-884
- Blueprint internal data
- Gainor JF et al., Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients with advanced RET-fusion+ non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 9008)
- Cocco E et al., NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018; 15(12): 731-747
- Lassen UN et al., Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann Oncol 2018; 29 (suppl_8): viii133-viii148
- Hong DS et al., Larotrectinib efficacy and safety in adult TRK fusion cancer patients. J Clin Oncol 37, 2019 (suppl; abstr 3122)
- Fernandez-Cuesta L et al., CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 2014; 4(4): 415-422
- Duruisseaux M et al., NRG1 fusion-positive lung cancers: clinicopathologic profile and treatment outcomes from a global multicenter registry. J Clin Oncol 37, 2019 (suppl; abstr 9081)
© 2019 Springer-Verlag GmbH, Impressum