Current insights into BTK inhibition and other targeted approaches in CLL
Treatment-naïve disease
Ibrutinib vs. watch & wait in asymptomatic patients
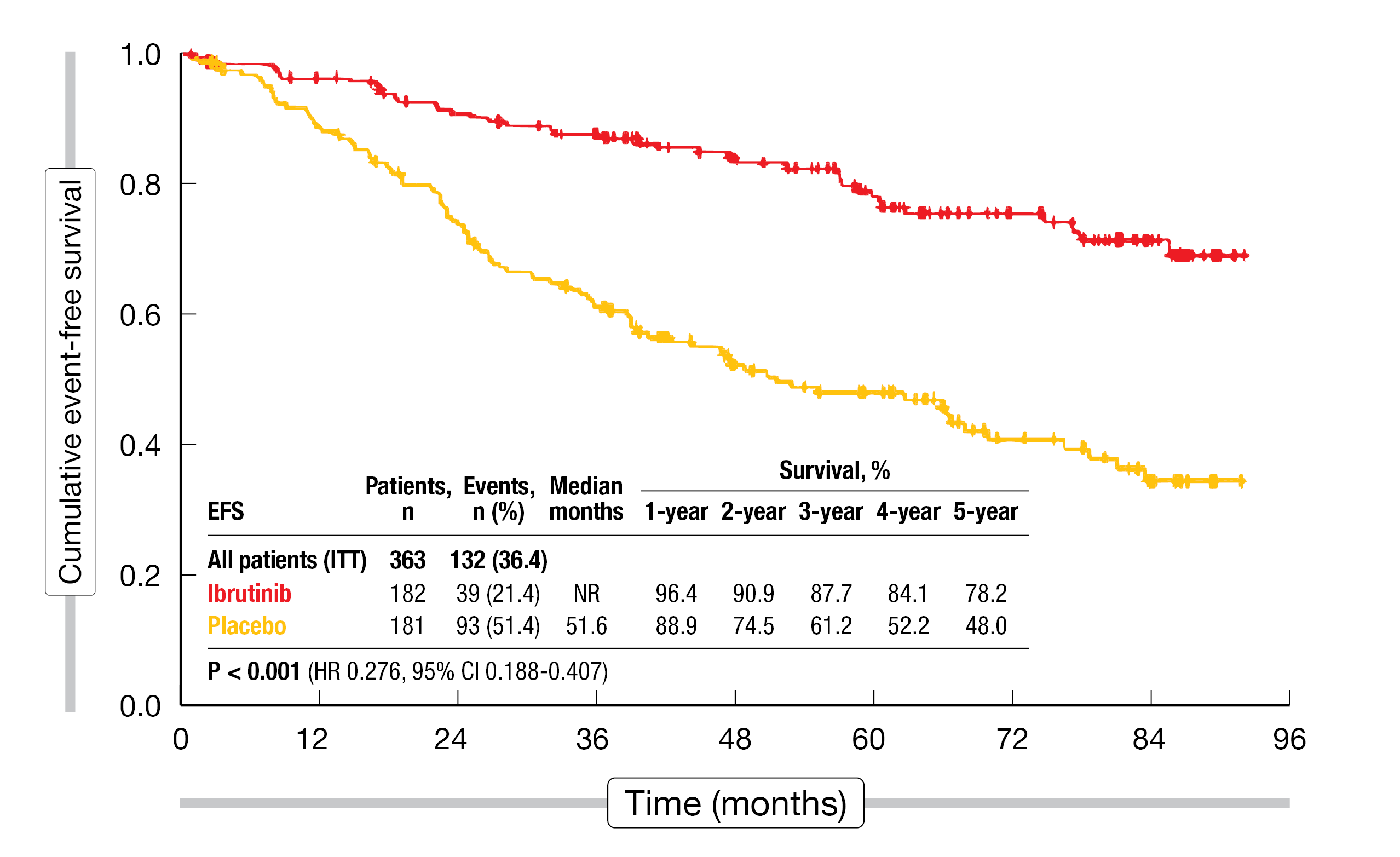
In the setting of early-stage, asymptomatic chronic lymphocytic leukemia (CLL), the concept of watch & wait in the era of targeted agents was challenged by the placebo-controlled, double-blind, phase III CLL12 study. This trial assessed the use of ibrutinib 420 mg OD (n = 182) vs. placebo (n = 181) until symptomatic disease progression in treatment-naïve patients with asymptomatic CLL Binet stage A who had an increased risk due to factors such as del(17p), IGHV mutation status, or age. A group of 152 CLL patients without increased risk constituted the watch & wait cohort. The primary endpoint analysis has demonstrated superior event-free survival (EFS) until symptomatic disease progression or death from any cause for the ibrutinib-treated patients [1]. At a median observation time of 69.3 months, Langerbeins et al. presented the final analysis of the CLL12 study at EHA 2023 [2].
The superiority of ibrutinib regarding EFS was confirmed with the prolonged follow-up: while median EFS had not been reached with ibrutinib, it was 51.6 months with placebo (HR, 0.276; p < 0.001; Figure 1). Likewise, median progression-free survival (PFS) had not been reached in the experimental arm and was 14.0 months in the control arm (HR, 0.174; p < 0.001). The overall response rate (ORR) was 72.5 % vs. 5 %. Only 15.9 % of ibrutinib-treated patients received subsequent therapy (vs. 43.6 %), and time to next treatment (TTNT) was significantly longer (not reached vs. 68.5 months; HR, 0.244; p < 0.001). For PFS2 that was assessed from the start of subsequent treatment, the analysis revealed no advantage of ibrutinib (16.6 vs. 35.1 months; HR, 1.471). Median overall survival (OS) had not been reached in either treatment arm (HR, 0.791; p = 0.562). Similarly, OS, as measured from CLL diagnosis to death, did not differ across the arms; here, the analysis also included the watch-and-wait cohort.
With respect to adverse events (AEs) of clinical interest, ibrutinib-treated patients experienced bleeding and cardiac arrhythmias in 36.5 % and 22.4 %, respectively. Fatal AEs of interest occurred in 2.4 % in the experimental arm. Second malignancies were reported in 12.9 % for ibrutinib vs. 21.4 % for placebo, with grade 5 events observed in 1.2 % vs. 3 %. In the watch & wait group, second malignancies emerged in 9.9 % and were the only AEs documented.
The authors noted in their summary that ibrutinib is associated with relevant cardiovascular toxicity and increased susceptibility to bleeding. While EFS, PFS and TTNT were significantly longer in ibrutinib-treated patients, this did not translate into an OS advantage compared to the placebo group where median OS was excellent. Therefore, watch & wait should remain the standard of care in asymptomatic CLL patients with high-risk features even in the era of targeted drugs.
Figure 1: CLL12 study: long-term event-free survival with ibrutinib vs. placebo in asymptomatic patients
CAPTIVATE: 4-year results from the FD cohort
First-line treatment with ibrutinib plus venetoclax was assessed in the international phase II CAPTIVATE study that contained two cohorts investigating MRD-guided randomized treatment discontinuation (MRD cohort) and fixed-duration treatment (FD cohort). Patients in the FD cohort received ibrutinib 420 mg OD for a total of 15 months including lead-in in addition to venetoclax 400 mg OD for 12 months. After the end of the fixed-duration period, patients could receive retreatment upon progression with single-agent ibrutinib or ibrutinib plus venetoclax as per investigator’s discretion. The primary analysis has shown an ORR of 96 %, a best undetectable minimal residual disease (uMRD) rate of 77 %, and a 24-month PFS rate of 95 % [3]. Ghia et al. presented 4-year follow-up data from the FD cohort (n = 159) after a median time on study of 49.8 months that included 36.0 months after the completion of FD therapy [4].
Ibrutinib/venetoclax continued to provide clinically meaningful benefits and deep, durable responses. Thirty-six months after the end of treatment (EOT), 44 % of patients still showed complete response (CR). Median duration of response had not been reached, which also applied to median duration of CR. Patients with del(17p)/TP53 mutation and unmutated IGHV showed a somewhat faster decline in CR over time, with 36-month rates of 30 % and 43 %, respectively. The 4-year PFS rate was high at 79 % in the entire group. For patients with del(17p)/TP53 mutation and unmutated IGHV, this was 63 % and 73 %, respectively. In the overall population, 21 % of patients had uMRD in the peripheral blood 36 months after EOT. The analysis according to MRD status demonstrated that the 48-month PFS rate was significantly higher in patients with uMRD than in those with detectable MRD 3 months after EOT (90 % vs. 66 %). Ninety-eight percent of all treated patients were alive at 4 years. Median TTNT had not been reached, and 84 % had not started their next treatment.
Nineteen patients who had progressed after completing the fixed-duration regimen in either the FD or the MRD cohort placebo arm initiated retreatment with single-agent ibrutinib, which yielded promising responses. One of 17 response-evaluable patients achieved CR, and 13 obtained partial remission. In the retreated group, no AEs occurred that prompted dose reduction or discontinuation. The overall safety profile was manageable and unchanged from that previously reported. In their summary, the authors pointed out that ibrutinib/venetoclax represents an effective all-oral, once-daily, chemotherapy-free fixed-duration regimen for untreated patients with CLL/small lymphocytic lymphoma (SLL).
Six-year update of CLL14: venetoclax/obinutuzumab
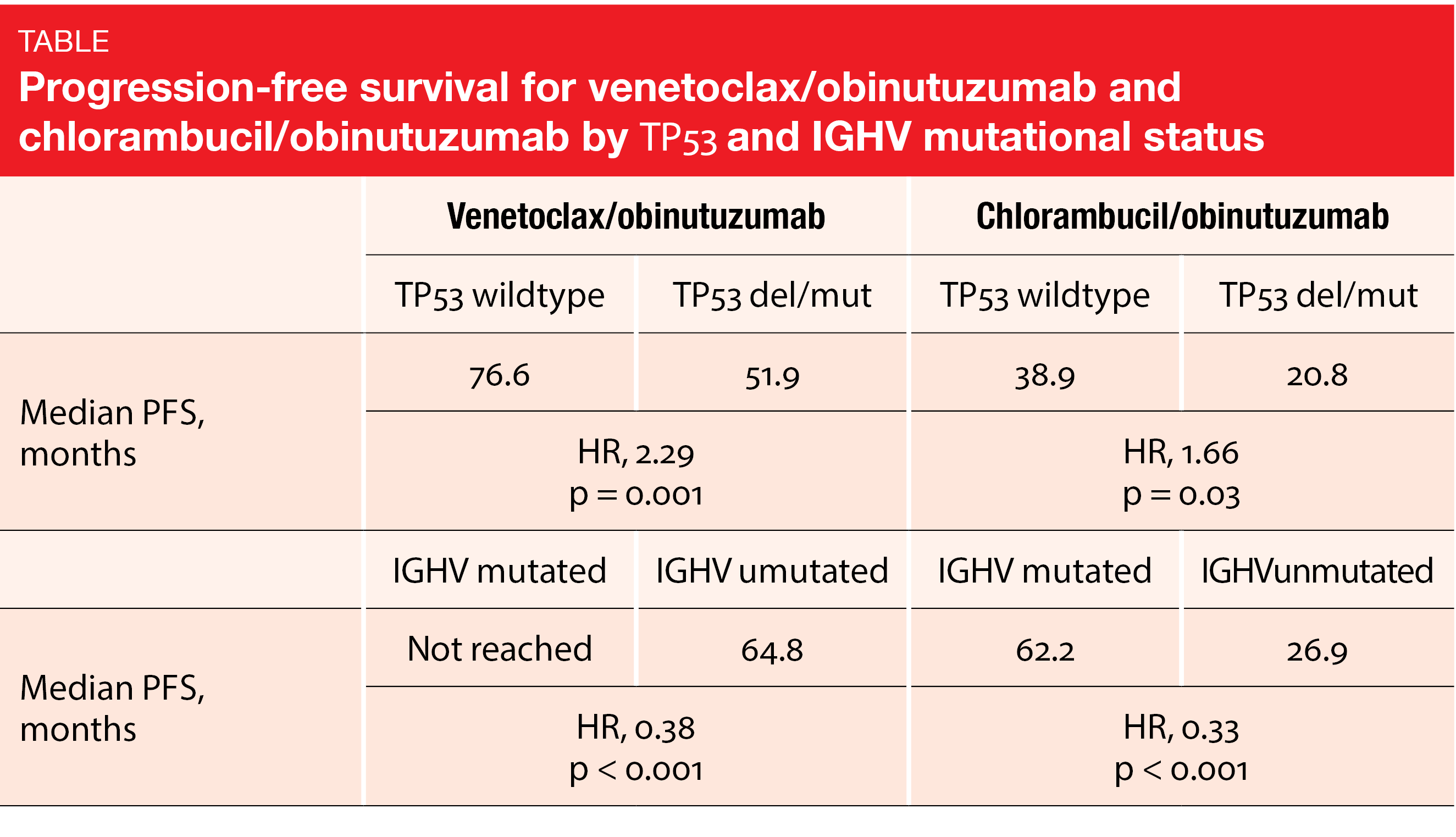
Fixed-duration treatment with venetoclax plus obinutuzumab was assessed by the randomized, open-label, phase III CLL14 study conducted in patients with previously untreated CLL who had coexisting medical conditions (CIRS > 6 and/or creatinine clearance < 70 mL/min). In the experimental arm (n = 216), venetoclax/obinutuzumab was administered for 6 cycles, which was followed by venetoclax monotherapy for another 6 cycles. Patients in the control arm (n = 216) received chlorambucil plus obinutuzumab for 6 cycles followed by chlorambucil for 6 cycles. The primary analysis revealed a significant PFS benefit for venetoclax/obinutuzumab vs. chlorambucil/obinutuzumab, with 24-month rates of 88.2 % vs. 64.1 % (HR, 0.35; p < 0.001) [5]. At EHA 2023, Al-Sawaf et al. reported an update of the CLL14 trial after a median of 76.4 months, with all patients being off study treatment for > 5 years [6]. Median PFS was 76.2 vs. 36.4 months for venetoclax/obinutuzumab vs. chlorambucil/obinutuzumab, which translated into a 60 % risk reduction (HR, 0.40; p < 0.0001). The 6-year PFS rates were 53.1 % vs. 21.7 %. High-risk characteristics including TP53 deletion/mutation and unmutated IGHV correlated with shorter PFS in both study arms. Nevertheless, patients with high-risk features fared considerably better when treated in the experimental arm, thus gaining several years of treatment-free disease control (Table). Within the venetoclax-treated group, the multivariate model identified lymph node size ≥ 5 cm, unmutated IGHV and TP53 deletion/mutation as independent negative prognostic factors for PFS.
Median TTNT had not been reached with venetoclax/obinutuzumab and was 52.9 months with chlorambucil/obinutuzumab (HR, 0.44; p < 0.0001). At the time of the analysis, more than 60 % of patients treated with venetoclax/obinutuzumab had not required any second-line treatment. Median OS had not been reached yet in either arm; at 6 years, the OS rates were 78.7 % vs. 69.2 % (HR, 0.69; p = 0.052). CLL-related mortality was lower with venetoclax/obinutuzumab (18.8 % vs. 37.1 %).
Longitudinal MRD assessments showed MRD < 10-4 rates of 7.9 % vs. 1.9 % five years after EOT. The data confirmed the prognostic significance of the EOT MRD status: the depth of remission at a cutoff of 10-4 correlated with OS (p < 0.001) and PFS. This provides the rationale for the implementation of MRD-guided strategies to further improve patient prognosis. The safety update yielded virtually no post-treatment toxicity in either arm. Second primary malignancies occurred in 14.2 % vs. 8.4 %, without any statistically significant difference in cumulative incidence across the arms.
SEQUOIA: zanubrutinib in elderly patients
The next-generation BTK inhibitor zanubrutinib was tested in the randomized, controlled, phase III SEQUOIA trial in treatment-naïve CLL/SLL patients who were ≥ 65 years or ≥ 18 years of age and unsuitable for treatment with chemoimmunotherapy. In Cohort 1 of the study, patients without del(17p) were treated with either zanubrutinib 160 mg BID until progression (n = 241) or bendamustine plus rituximab (BR) for 6 cycles (n = 238), while the patients in Cohort 2 had del(17p) and received zanubrutinib monotherapy (n = 111). After a median follow-up of 26.2 months, the study met its primary endpoint, with superior PFS results for zanubrutinib in Cohort 1 and similar outcomes in Cohort 2 [7]. Updated findings were reported by Shadman et al. at ICML 2023 after 18 months of additional follow-up [8].
In Cohort 1, the risk of progression or death was reduced by 70 % with zanubrutinib compared to BR (median PFS, not reached vs. 42.2 months; HR, 0.30; p < 0.0001). At 42 months, 82.4 % vs. 50.0 % of patients were alive and progression-free. The rate of complete remissions/CR with incomplete hematologic recovery (CRi) was 17.4 % in the experimental arm, indicating deepening of response as it represented an increase versus the rate achieved at the primary analysis. Zanubrutinib therapy, as compared to BR, induced significant PFS benefits in patients with both mutated IGHV (not reached vs. 49.4 months; HR, 0.35; p = 0.00033) and unmutated IGHV (not reached vs. 33.7 months; HR, 0.23; p < 0.0001). For OS, the 42-month rates were 89.4 % vs. 88.3 % (HR, 0.87).
Similar results were observed for zanubrutinib in the patients with del(17p) included in Cohort 2. The 42-month rates for PFS and OS were 79.4 % and 89.5 %, respectively. Again, at 14.5 %, the CR/CRi rate was improved compared to the primary analysis. Zanubrutinib was well tolerated, and the AEs reported were in keeping with the known safety profile of BTK inhibitors. The rates of atrial fibrillation or flutter remained low over time. Taken together, the extended follow-up of the SEQUOIA study demonstrated sustained benefits of zanubrutinib, supporting the first-line use of this agent in the elderly, in patients with comorbidities, and in those with del(17p).
MRD findings for zanubrutinib/obinutuzumab/venetoclax
In the phase II setting, the combination of zanubrutinib with obinutuzumab/venetoclax (BOVen) for 8–24 months was evaluated in 52 patients with previously untreated CLL/SLL. The primary endpoint of the study was defined by uMRD < 10-4 in the peripheral blood on two consecutive occasions, which was confirmed by bone marrow assessment. These patients discontinued treatment. BOVen appeared well-tolerated and led to high uMRD rates according to the primary analysis [9]. Updated findings were reported at ICML 2023 by Soumerai et al. [10].
After a median follow-up of 40 months, uMRD as best/EOT response was found in the peripheral blood in 96 % and in both blood and marrow in 92 %. All patients discontinued the treatment after a median of 10 months including the 2-month lead-in. Median PFS had not been reached yet at the time of the analysis. No additional safety signals were reported during the long-term observation period. In patients with uMRD in the bone marrow (n = 46), median MRD-free survival with the BOVen regimen was 29.8 months.
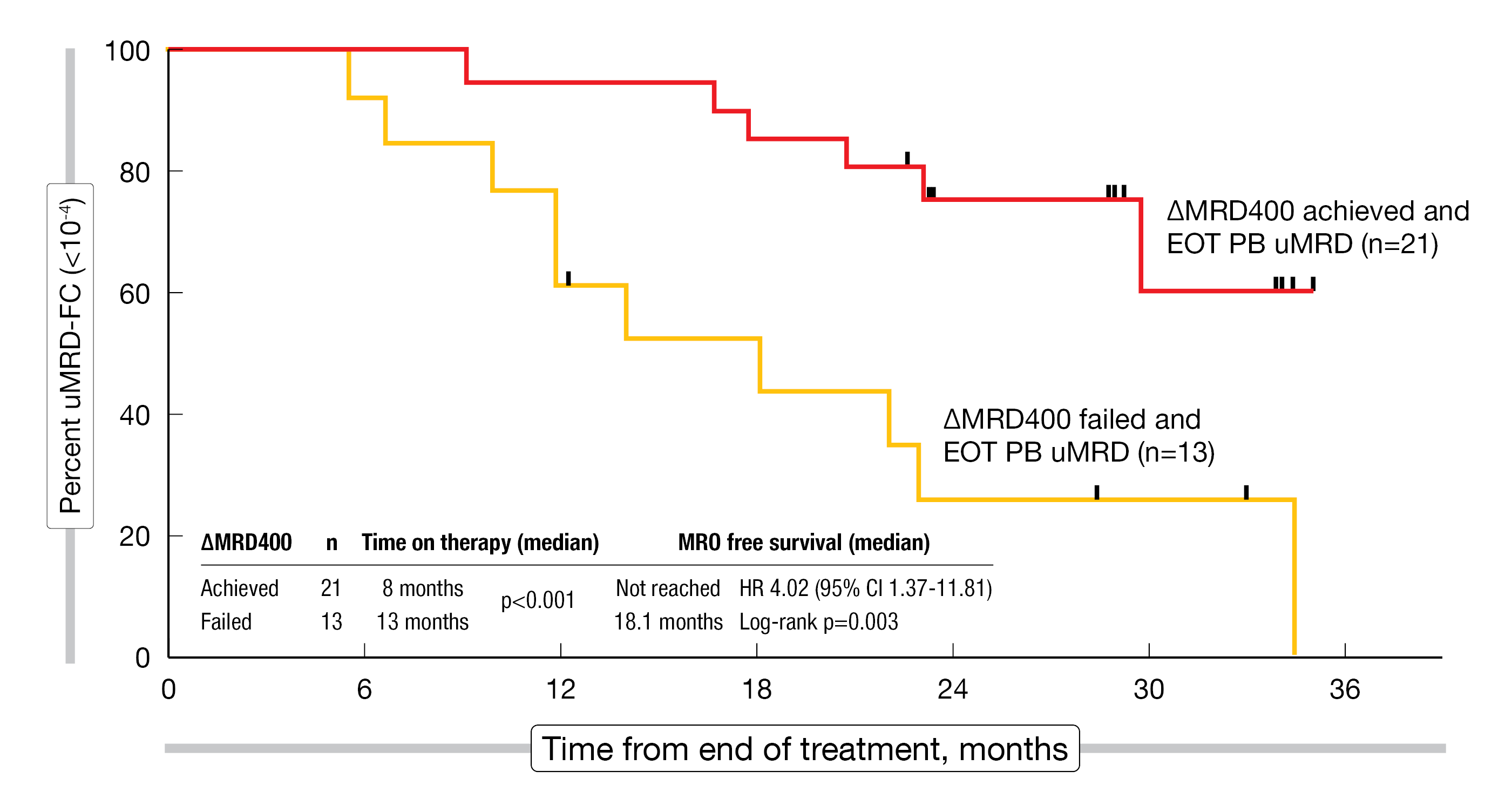
ΔMRD400, which represents a ≥ 400-fold reduction in peripheral blood MRD baseline levels until day 1 of cycle 5, was achieved by 60 % of patients. All of them developed early uMRD in the bone marrow after a median of 6 months. In the group of patients who did not achieve ΔMRD400, the time to uMRD in the bone marrow was 11 months; only 21 % developed uMRD within 8 months. Interestingly, ΔMRD400 did not appear to concur with traditional risk factors such as TP53 or IGHV mutational status. With longer follow-up, ΔMRD400 correlated with longer MRD-free survival despite shorter time on therapy (Figure 2). A phase II trial is exploring ΔMRD400-directed first-line treatment with BOVen for 24 vs. 10 months based on the hypothesis that longer duration of therapy in patients who failed to achieve ΔMRD400 will further improve uMRD duration.
Figure 2: Association of ΔMRD400 with longer MRD-free survival in patients treated with the BOVen regimen.
EOT, end of treatment; PB, peripheral blood
Relapsed/refractory disease
Venetoclax/rituximab: 7-year data from MURANO
Fixed-duration treatment in the relapsed/refractory setting was evaluated in the global, randomized, open-label phase III MURANO trial. Patients in the experimental arm received venetoclax 400 mg OD for 2 years plus rituximab for 6 months, while the control arm was treated with BR for 6 months. Venetoclax/rituximab, as compared to BR, gave rise to significantly improved PFS, with 2-year PFS rates of 84.9 % vs. 36.3 % (HR, 0.17; p < 0.001) [11]. At EHA 2023, Kater et al. reported the final long-term analysis [12].
After a follow-up of 7 years, venetoclax/rituximab induced sustained improvements of the survival endpoints compared to BR. Median PFS was 54.7 vs. 17.0 months (HR, 0.23; p < 0.0001), while median OS had not been reached in the experimental arm and was 87.8 months in the control arm (HR, 0.53; p < 0.0002). At 7 years, 69.6 % vs. 51.0 % of patients were alive, and 23.0 % vs. 0 % were progression-free. Moreover, the experimental treatment gave rise to significantly prolonged TTNT (63.0 vs. 24.0 months; HR, 0.30; p < 0.0001). No new safety signals have been identified since the 5-year analysis [13].
The MRD status at EOT was evaluable in 118 patients. Most of those who received the full 2 years of venetoclax/rituximab treatment achieved uMRD at EOT (n = 83). MRD conversion with subsequent disease progression did not occur until approximately 4 years after EOT in this cohort, with a median time to conversion of 19.4 months and a median time from conversion to progression of 28.3 months. In the group that completed 2 years of venetoclax without disease progression, uMRD was predictive of prolonged PFS: median PFS since EOT was more than 11 times longer among those with uMRD than in the cohort with high MRD (52.5 vs. 4.6 months; HR, 17.22; p < 0.0001). For OS since EOT, the same analysis revealed a trend. Fourteen patients showed sustained uMRD after EOT at the most recent follow-up. This group was enriched for the favorable baseline characteristics TP53 wildtype and mutated IGHV.
A substudy of the MURANO trial explored retreatment following disease progression. Twenty-five patients most of whom had high-risk features were retreated with fixed-duration venetoclax/rituximab after a median of 2.3 years from the final study drug dose. In this group, the best ORR was as high as 72.0 %, including a CR rate of 24 %. Median PFS was 23.3 months, and median OS had not been reached. uMRD at the end of venetoclax/rituximab retreatment resulted in 32 % but was not sustained in the long run. The authors concluded that venetoclax/rituximab retreatment remains a viable option for patients with relapsed/refractory CLL. Overall, the long-term data from MURANO continue to support the use of fixed-duration venetoclax/rituximab and demonstrate the feasibility of venetoclax retreatment in the context of fixed-duration strategies.
Vision: time-limited venetoclax and ibrutinib
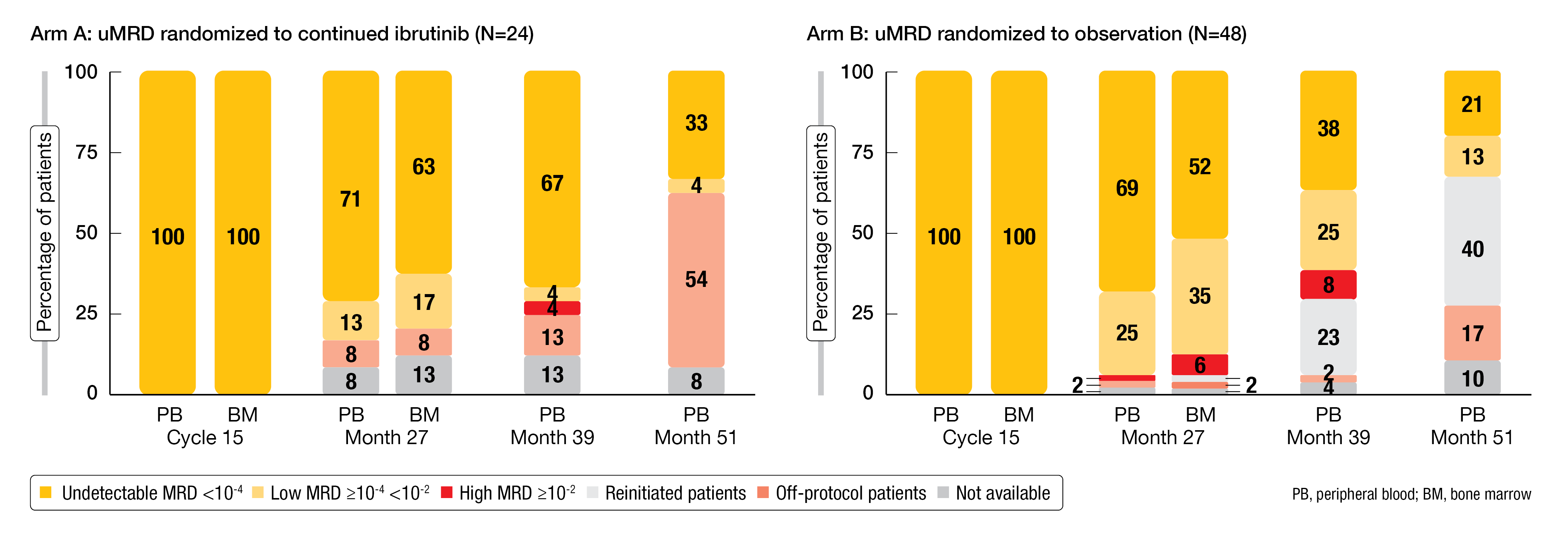
The aim of the Vision/HO141 trial is the assessment of MRD-guided treatment in all-comer patients with relapsed/refractory CLL. After induction therapy with ibrutinib/venetoclax for 15 cycles (n = 225), patients who achieved at least partial response and uMRD at cycle 15 (n = 72; 32 %) were randomized to either ibrutinib maintenance until progression (arm A; n = 24) or observation (arm B; n = 48). Patients in arm B who developed CLL progression or became MRD-positive (> 10-2) received retreatment with ibrutinib until progression and venetoclax for 12 months. Those who remained MRD-positive after the 15-cycle induction period were not randomized and went on to receive ibrutinib until progression (n = 153). The primary analysis has shown a favorable benefit-risk profile of MRD-based cessation and reinduction; the primary endpoint of PFS at 12 months after stopping treatment in arm B was met (98 %) [14]. Kater et al. reported the long-term outcome and MRD kinetics after a median follow-up of 50.8 months at EHA 2023 [15].
At 51 months, the PFS rates were 92 % for arm A that received ibrutinib maintenance, 81 % for arm B that was treated based on MRD, and 75 % for the non-randomized cohort. TTNT rates for these groups were 92 %, 96 % and 88 %, respectively. Likewise, arms A and B fared better than the non-randomized cohort with respect to OS, with 51-month rates of 96 %, 92 %, and 84 %, respectively.
At 51 months, uMRD was present in 33 % and 21 % in arms A and B, respectively (Figure 3), and in 22 % in the non-randomized cohort. In arm B, 40 % of initially MRD-negative patients experienced MRD conversion (> 10-2) after a median of 24 months since the start of observation. These patients showed an enrichment for TP53 aberrations and genomic complexity (i.e., ≥ 3 aberrations). Retreatment with 12 cycles of ibrutinib/venetoclax led to second uMRD responses in 47 %, while progression was only observed in one patient (5 %). The authors concluded that treatment cessation with MRD-guided reinitiation is a feasible approach in patients with relapsed/refractory CLL who have achieved uMRD after 15 cycles of induction treatment.
Figure 3: Changes in MRD responses over time after 15 cycles of induction with venetoclax/ibrutinib
Zanubrutinib in patients intolerant to BTK inhibitors
Zanubrutinib has been designed to maximize BTK occupancy and minimize off-target kinase binding and associated AEs [16]. Patients with B-cell malignancies who are intolerant of other BTK inhibitors are being treated with zanubrutinib in the ongoing, single-arm, phase II BGB-3111-215 study. Cohort 1 is intolerant of ibrutinib (n = 44), while Cohort 2 shows intolerance of acalabrutinib (n = 9) or both ibrutinib and acalabrutinib (n = 8). Previously published results have demonstrated that zanubrutinib is effective and well tolerated in this setting [17]. At ICML 2023, Shadman et al. reported preliminary longer-term findings in patients with CLL/SLL after a median follow-up of 28.2 and 10.1 months in Cohorts 1 and 2, respectively [18]. In both cohorts, > 65 % of patients remained on treatment. The median zanubrutinib treatment duration was 27.1 and 8.1 months, respectively.
With zanubrutinib, 68.4 % of ibrutinib-intolerance AEs and 71.4 % of acalabrutinib-intolerance AEs did not recur. No intolerance AE recurred with increased severity; among those that did recur, 73.3 % of ibrutinib-intolerance AEs and 33.3 % of acalabrutinib-intolerance AEs recurred at lower grades. The most common grade ≥ 3 treatment-emergent AE (TEAE) on zanubrutinib was neutropenia (11.5 %). TEAEs leading to dose interruption and reduction occurred in 49.2 % and 24.6 %, respectively.
In terms of efficacy, the analysis revealed an ORR of 71.9 % and a disease control rate of 94.7 % across both cohorts. At 12 months, 88.3 % of patients were progression-free. In their summary, the authors concluded that ibrutinib- and acalabrutinib-intolerant patients are likely to benefit from a switch to zanubrutinib, which appears to be a viable option in this setting.
HRQoL data from the ALPINE study
Zanubrutinib 160 mg BID (n = 327) was compared to ibrutinib 420 mg OD (n = 325) until progression in patients with relapsed/refractory CLL/SLL in the randomized, open-label phase III ALPINE study. At the time of the final PFS analysis, zanubrutinib was significantly superior to ibrutinib regarding PFS (HR, 0.65; p = 0.0024) and ORR (86.2 % vs. 75.7 %; p = 0.0007) [19]. Eichhorst et al. presented data on health-related quality of life (HRQoL), which was a secondary objective of the study, at EHA 2023 [20]. Patient-reported outcomes were assessed using the EORTC QLQ-C30 questionnaire and the EuroQoL visual analog scale (EQ-VAS).
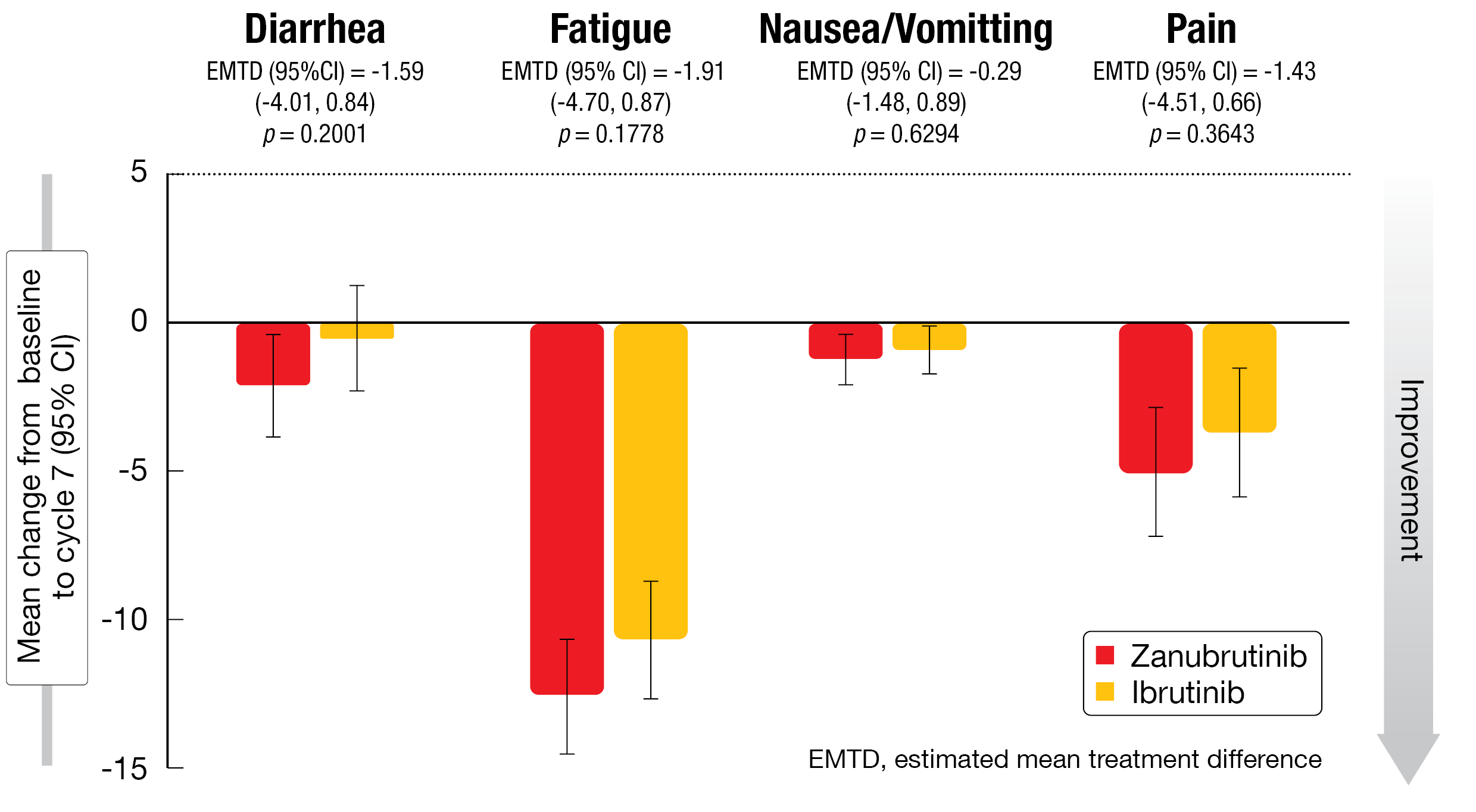
Global health status as well as physical and role functioning improved in both arms from baseline to both cycle 7 and 13. All improvements were clinically meaningful for the zanubrutinib arm, although no clinically meaningful differences resulted across the two arms by cycle 13. Regarding the symptom scales, both arms showed decreases in fatigue and pain (Figure 4). Zanubrutinib gave rise to clinically meaningful improvements for both of these symptoms at cycles 7 and 13. For diarrhea, zanubrutinib-treated patients experienced more pronounced improvement, although this difference did not reach the predefined clinically relevant threshold. The mean change from baseline in the EQ-VAS demonstrated a similar pattern of improvement with both agents up to cycle 13.
Overall, the data suggested that treatment with zanubrutinib improved HRQoL over time, although the differences were not significant given the generally good HRQoL at baseline in both arms. Long-term follow-up and additional analyses linking patient-reported endpoints to clinical outcomes will further determine the effect of zanubrutinib on HRQoL.
Figure 4: Mean changes in EORTC QLQ-C30 symptom scales from baseline at cycle 7 with zanubrutinib vs. ibrutinib
Findings in the Chinese subgroup of ALPINE
Patients included in the ALPINE study were stratified by geographical region. Qiu et al. reported a descriptive analysis of the prespecified subgroup from China that included 90 patients 47 of whom were treated with zanubrutinib [21]. At a median follow-up of 25.3 months, zanubrutinib, as compared to ibrutinib, significantly improved PFS, with 18-month rates of 88.9 % vs. 71.6 % (HR, 0.24; p = 0.002). In the high-risk group featuring del(17p)/TP53 mutation, the patients in the experimental arm derived a 49 % risk reduction (18-month PFS rates, 80.0 % vs. 64.3 %; HR, 0.51). ORR also favored zanubrutinib (87.2 % vs. 76.7 %). The efficacy results were consistent with those from the global population. Zanubrutinib showed a favorable safety profile, with lower treatment discontinuation rates compared to ibrutinib (14.9 % vs. 41.9 %), as well as lower rates of grade ≥ 3 AEs (64.4 % vs. 72.1 %) and serious AEs (35.6 % vs. 51.2 %).
Resistance mechanisms to pirtobrutinib
Patients receiving covalent BTK inhibitors (cBTKi) frequently discontinue treatment due to progression or intolerance [22-24]. BTK cysteine 481 substitution has been found to contribute to acquired cBTKi resistance in the context of treatment with ibrutinib, acalabrutinib and zanubrutinib [25, 26]. Non-covalent BTK inhibitors use a different BTK-binding mechanism that might provide benefit in cBTKi-resistant patients [27]. The non-covalent BTK inhibitor pirtobrutinib has been shown to inhibit both wildtype and C481-mutant BTK with equal low nM potency [28, 29]. Brown et al. investigated the genomic evolution and resistance to pirtobrutinib in cBTKi-pretreated CLL patients enrolled in the phase I/II BRUIN study (n = 279) [30]. Next-generation sequencing results for paired baseline and progression samples were available from 49 patients who progressed on pirtobrutinib.
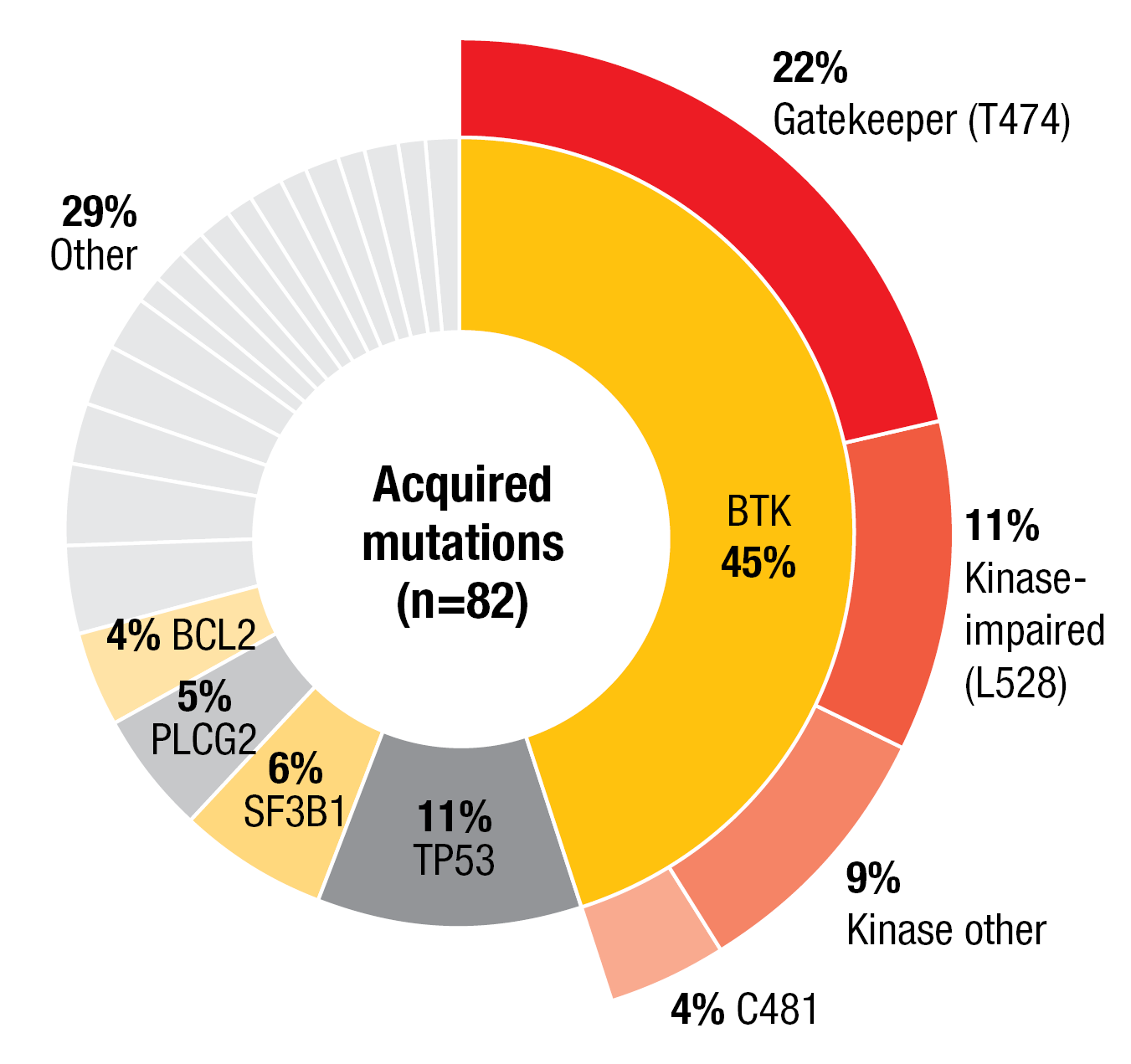
The most common mutations at baseline were found in BTK (51 %), TP53 (49 %), ATM (27 %), NOTCH1 (20 %), SF3B1 (18 %), and PLCG2 (10 %). A total of 31 BTK mutations in 25 patients were detected at baseline, including C481S (n = 23), C481R (n = 4), C481Y (n = 2), C481F (n = 1), and T474I (n = 1). At the time of progression on pirtobrutinib, acquired mutations tended to be non-C481 BTK mutations, with the gatekeeper T474 and kinase-impaired L528 mutations representing the greatest proportion (Figure 5). Approximately half of progressing patients had not acquired BTK mutations or any mutations at all, which suggests alternate resistance mechanisms.
In 24 %, acquired non-C481 mutations observed at disease progression had pre-existed at low variant allele frequencies of 1–3 % at baseline; this is indicative of emergence on prior cBTKi treatment. C481 clones decreased or disappeared in 92 % of patients. Pirtobrutinib displayed efficacy regardless of the type of prior cBTKi as well as baseline and acquired BTK mutations, with ORRs ranging from 83 % to 92 % across several groups with different acquired BTK mutations.
Figure 5: Pattern of acquired resistance mutations to pirtobrutinib
Treatment persistence and surrogate trial endpoints
Persistence with oral therapy is a well-recognized factor for the efficacy of treatment [31-33]. Real-world insights regarding persistence with oral BTK inhibitors and the impact of comorbidities on this factor in Swedish patients with CLL/SLL were presented at EHA by Nevalainen et al. [34]. The analysis was based on pseudonymized data collected in the National Prescribed Drugs Register and the National Patient Register from January 2017 to December 2021.
The researchers identified patients with CLL (n = 1,425) and SLL (n = 115) who had received new prescriptions of oral CLL drugs for continuous use. Prescription data related to ibrutinib, acalabrutinib, idelalisib, and venetoclax. Among these, ibrutinib was prescribed for the majority of cases (87 %), with a median treatment time of 1.4 years. Venetoclax was the second most commonly prescribed drug, followed by acalabrutinib and idelalisib. The most common change in treatment was from ibrutinib to venetoclax, the second most common from ibrutinib to acalabrutinib.
With respect to drug persistence, the analysis revealed 36-month rates of 50 % and ≤ 10 % for ibrutinib and idelalisib, respectively. The 50 % persistence for idelalisib was reached at approximately 10 months. For acalabrutinib, the follow-up was very short; at 9 months, the persistence rate was 92 %. Comorbidities that mostly included infections and cardiovascular disease were present in 62 % of patients at the time of or after their first prescription. While the presence of both infections and cardiovascular disease significantly increased the likelihood of ibrutinib discontinuation, venetoclax discontinuation was more likely in the context of infections but not in the context of cardiovascular disease. For acalabrutinib and idelalisib, no statistically significant difference was seen among any of the comorbidity groups. The authors concluded that for CLL patients treated with an oral drug for continuous use, persistence with therapy may reflect adherence and possibly function as a proxy for PFS.
Bahar et al. conducted a systematic literature review of 69 randomized controlled trials to evaluate the validity of ORR as a surrogate endpoint for PFS and OS, and PFS as a surrogate endpoint for OS in CLL, in light of the need to identify surrogate outcomes that can be measured earlier in clinical trials than the true endpoints [35]. Indeed, the scientists found robust evidence that ORR serves as a surrogate for PFS in CLL, especially when evaluating the treatment effect of BTK inhibitors. Some evidence was obtained for an association between PFS and OS, while no clear evidence of ORR as a surrogate for OS emerged.
REFERENCES
- Langerbeins P et al., The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood 2022; 139(2): 177-187
- Langerbeins P et al., Ibrutinib versus placebo in patients with asymptomatic, treatment-naïve early stage CLL. EHA 2023, abstract S200
- Tam CS et al., Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood 2022; 139(22): 3278-3289
- Ghia P et al., Fixed-duration ibrutinib + venetoclax for first-line treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma: 4-year follow-up from the FD cohort of the phase 2 CAPTIVATE study. ICML 2023, abstract 155
- Fischer K et al., Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019; 380: 2225-2236
- Al-Sawaf O et al., Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized CLL14 study. EHA 2023, abstract S145
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomized, controlled, phase 3 trial. Lancet Oncol 2022; 23(8): 1031-1043
- Shadman M et al., Zanubrutinib vs bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: extended follow-up of the SEQUOIA study. ICML 2023, abstract 154
- Soumerai JD et al., Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol 2021; 8(12): e879-e890
- Soumerai JD et al., Long-term follow-up of multicenter phase II trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated patients with CLL/SLL. ICML 2023, abstract 153
- Seymour JF et al., Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018; 378(12): 1107-1120
- Kater AP et al., MURANO: final 7-year follow up and retreatment analysis in venetoclax-rituximab-treated patients with relapsed/refractory chronic lymphocytic leukemia. EHA 2023, abstract S201
- Seymour JF et al., Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022; 140(8): 839-850).
- Kater AP et al., Minimal residual disease-guided stop and start of venetoclax plus ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia (HOVON141/VISION): primary analysis of an open-label, randomised, phase 2 trial. Lancet Oncol 2022; 23(6):818-828
- Kater AP et al., Time-limited venetoclax and ibrutinib for patients with relapsed/refractory CLL who have undetectable MRD 4-year follow up from the randomized phase II Vision/HO141 trial. EHA 2023, abstract S148
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940
- Shadman M et al., Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single-arm study. Lancet Haematol 2023; 10(1): e35-e45
- Shadman M et al., A phase 2 study of zanubrutinib in previously treated B-cell malignancies intolerant to ibrutinib and/or acalabrutinib: preliminary results from patients with CLL/SLL. ICML 2023, abstract 345
- Brown JR et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2023; 388(4): 319-332
- Eichhorst B et al., Zanubrutinib vs ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: impact on health-related quality of life. EHA 2023, abstract P640
- Qiu L et al., Improved efficacy and safety of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia in China: A subgroup of ALPINE. ICML 2023, abstract 592
- Woyach JA et al., BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol 2017; 35(13): 1437-1443
- Barr PM et al., Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv 2022; 14; 6(11): 3440-3450
- Seymour JF et al., Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in ELEVATE-RR. Blood 2023 Jun 30; blood.2022018818
- Estupiñán HY et al., BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib. Leukemia 2021; 35(5): 1317-1329
- Handunnetti SM et al., Three year update of the phase II ABT-199 (venetoclax) and ibrutinib in mantle cell lymphoma (AIM) study. Blood 2019; 134 (Supplement_1): 756
- Wang E et al., Mechanisms of resistance to noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med 2022; 386(8): 735-743
- Gomez EB et al., Pirtobrutinib preclinical characterization: a highly selective, non-covalent (reversible) BTK inhibitor. Blood 2023 Feb 16; blood.2022018674
- Aslan B et al., Pirtobrutinib inhibits wild-type and mutant Bruton’s tyrosine kinase-mediated signaling in chronic lymphocytic leukemia. Blood Cancer J 2022; 12(5): 80
- Brown JR et al., Genomic evolution and resistance to pirtobrutinib in covalent BTK-inhibitor pre-treated chronic lymphocytic leukemia patients: results from the phase I/II BRUIN study. EHA 2023, abstract S146
- Hohneker J et al., Perspectives on adherence and persistence with oral medications for cancer treatment. J Oncol Pract 2011; 7(1): 65-67
- Puts MTE et al., Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol 2014; 25(3): 564-577
- Paolella GA et al., Adherence to Oral Anticancer Medications: Evolving Interprofessional Roles and Pharmacist Workforce Considerations. Pharmacy (Basel) 2018; 6(1): 23
- Nevalainen E et al., Persistence with oral therapy as reflection of actual compliance for chronic lymphocytic leukemia – a national prescribed drug and patient register study from Sweden. EHA 2023, abstract S314
- Bahar N et al., A meta-analytic endpoint validation of surrogates used in clinical trials evaluating the efficacy of therapies in patients with chronic lymphocytic leukemia. ICML 2023, abstract 594
© 2023 Springer-Verlag GmbH, Impressum
More posts
DLBCL: treatment of elderly patients and relapsed disease
DLBCL: treatment of elderly patients and relapsed disease POLAR BEAR: novel regimen fr
Waldenström macroglobulinemia: findings from ASPEN and BRUIN
Waldenström macroglobulinemia: findings from ASPEN and BRUIN ASPEN: HRQoL for zanubrut
Outcome improvements in relapsed and untreated marginal zone lymphoma
Outcome improvements in relapsed and untreated marginal zone lymphoma Final analysis o
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors Mosun
Current insights into BTK inhibition and other targeted approaches in CLL
Current insights into BTK inhibition and other targeted approaches in CLL Treatment-na
Preface – ASCO/EHA/ICML 2023
Preface – ASCO/EHA/ICML 2023 © Dirk Gillissen – Arnon P. Kater, MD, PhD, Department o