Outcome improvements in relapsed and untreated marginal zone lymphoma
Final analysis of MAGNOLIA
Systemic treatment for patients with advanced marginal zone lymphoma (MZL) is often based on regimens used in follicular lymphoma, although new agents and combinations are called for. As MZL depends on B-cell receptor signaling, treatment with BTK inhibitors is being investigated in clinical trials. The multicenter, open-label, single-arm, phase II MAGNOLIA study was initiated to assess the potent and highly specific next-generation BTK inhibitor zanubrutinib 160 mg BID as monotherapy in patients with relapsed/refractory MZL after ≥ 1 CD20-directed regimen. Overall response rate (ORR) by independent review constituted the primary endpoint, which was met at the primary analysis after a median follow-up of 15.7 months [1]. At EHA 2023, Opat et al. presented the final analysis of MAGNOLIA after a median follow-up of 28 months [2]. A total of 68 patients had been enrolled 34 of whom were still receiving zanubrutinib.
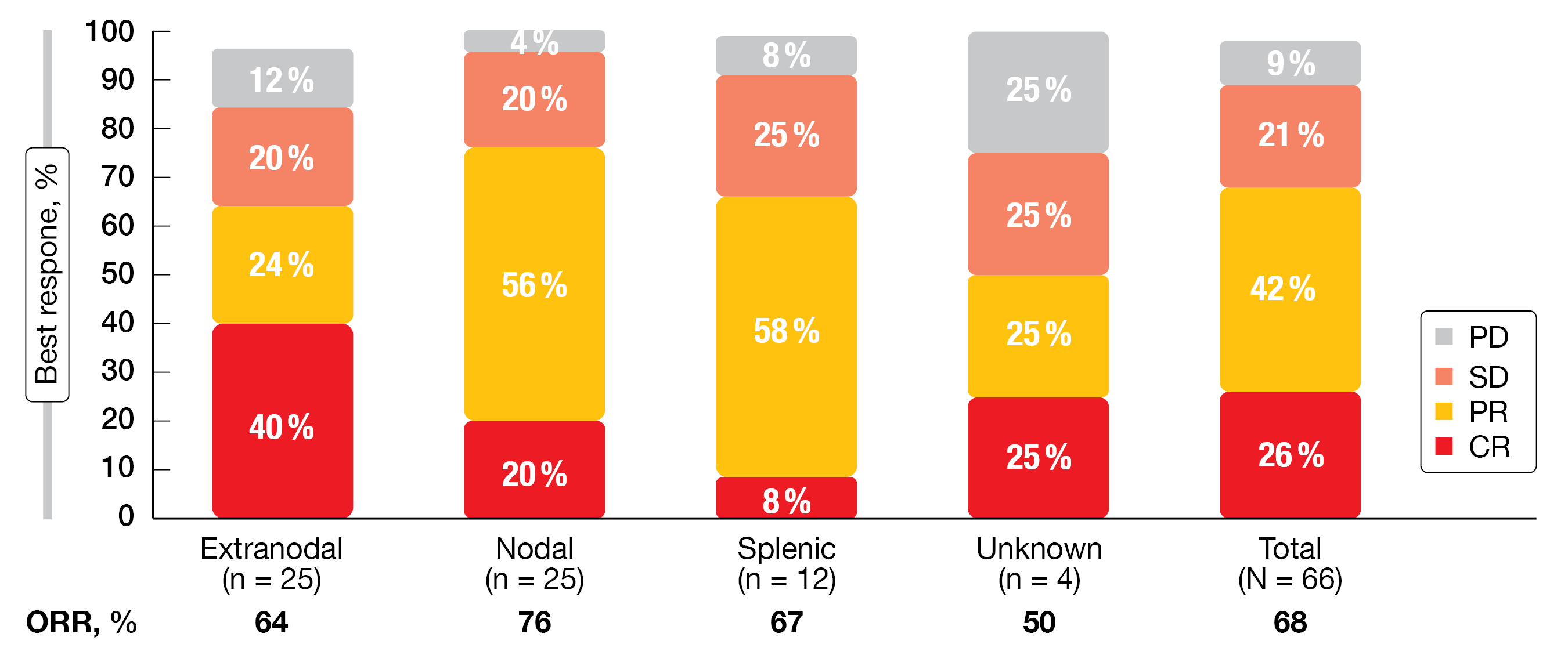
The ORR by independent review was 68 %, with 26 % of patients experiencing complete response (CR). All MZL subtypes (i.e., extranodal, nodal, splenic) showed high ORRs, with the highest ORR observed in patients with nodal MZL and the highest CR in those with extranodal MZL (Figure 1). Responses resulted in all key subgroups including difficult-to-treat cohorts. At 24 months, 86 % of patients were alive, and 71 % were progression-free. For duration of response, the 24-month rate was 73 %. Durable disease control was achieved in all three MZL subtypes.
Zanubrutinib was generally well tolerated. Treatment-emergent adverse events (TEAEs) of clinical interest mainly included infections (56 %) and hemorrhage (41 %), with grade ≥ 3 rates of 22 % and 1.5 %, respectively. Cardiac TEAEs were rare; all-grade hypertension, atrial fibrillation/flutter and ventricular extrasystoles occurred in 4 %, 3 %, and 1.5 %, respectively. The incidence of cardiac TEAEs was comparable to that observed in a pooled safety analysis of 10 clinical studies of zanubrutinib, and was lower than the rates reported for ibrutinib [3]. Overall, these data support the use of zanubrutinib in patients with relapsed/refractory MZL.
Figure 1: Best overall responses achieved with zanubrutinib by MZL subtype.
PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response
Comparisons of zanubrutinib with ibrutinib and rituximab
In the absence of head-to-head randomized controlled trials, matching-adjusted indirect comparisons were performed to estimate the comparative efficacy of zanubrutinib vs. ibrutinib and rituximab in the setting of relapsed/refractory MZL. The researchers obtained data for zanubrutinib from the MAGNOLIA trial [1] and the phase I/II BGB-3111-AU-003 study [4]. Regarding ibrutinib and rituximab, the analyses were based on data from the phase II PCYC-1121 trial [5, 6] and the phase III CHRONOS-3 trial, respectively [7]. Propensity score models were employed to match baseline characteristics, and prognostic factors were ranked by clinical experts. Variables balanced in the base-case model included number of prior lines of treatment, MZL subtype, response to prior therapy, and age. Additional variables such as bulky disease, prior anti-CD20 therapy and bone marrow involvement were considered in the sensitivity analysis conducted for zanubrutinib vs. ibrutinib; for zanubrutinib vs. rituximab, this was not possible due to a lack of reporting of additional factors from CHRONOS-3. Nevertheless, the impact of each covariate was explored via a leave-one-out analysis for both comparisons. Logistic regression models for binary outcomes and Cox proportional hazard models for time-to-event outcomes were used to estimate relative treatment effects.
The matching-adjusted indirect comparison for zanubrutinib vs. ibrutinib showed that zanubrutinib gave rise to a significant reduction in the risk of progression (adjusted HR, 0.38; p = 0.001) and a significantly higher ORR (OR, 2.37 according to the base-case model with all covariates; p < 0.01) [8]. Overall survival (OS) was comparable between the two treatments, which matches expectations for indolent lymphomas (adjusted HR, 0.68; p = 0.295), although point estimates favored zanubrutinib. The sensitivity analysis accounting for additional prognostic factors suggested that zanubrutinib and ibrutinib were comparable across all outcomes, owing in part to the low effective sample size for zanubrutinib in the expanded models, although point estimates were in favor of zanubrutinib.
Likewise, compared to rituximab, zanubrutinib therapy significantly reduced the risk of progression (adjusted HR, 0.29; p = 0.003) [9] and conferred significantly higher probability of response (OR, 5.09 according to the base-case model with all covariates; p < 0.01). OS was again comparable for the two drugs, while point estimates were in favor of zanubrutinib.
MALIBU: ibrutinib plus rituximab
In the untreated setting, the phase II IELSG47/MALIBU trial is aiming to assess the combination of ibrutinib and rituximab in patients with extranodal MZL. Moreover, a limited number of non-extranodal cases was included for a preliminary exploration of the safety and activity of ibrutinib plus rituximab in these subtypes, where data are lacking to date. The splenic and nodal MZL cohorts contained 30 and 15 patients, respectively. Thieblemont et al. presented the findings for these subgroups at ICML 2023 after a median follow-up of 23 months [10]. This is the first report for rituximab plus ibrutinib in the first-line setting of MZL. Rituximab was administered for a total of 8 infusions together with ibrutinib 560 mg OD for 2 years. The CR rate at 12 months and the progression-free survival (PFS) rate at 5 years constituted the two primary endpoints of the study.
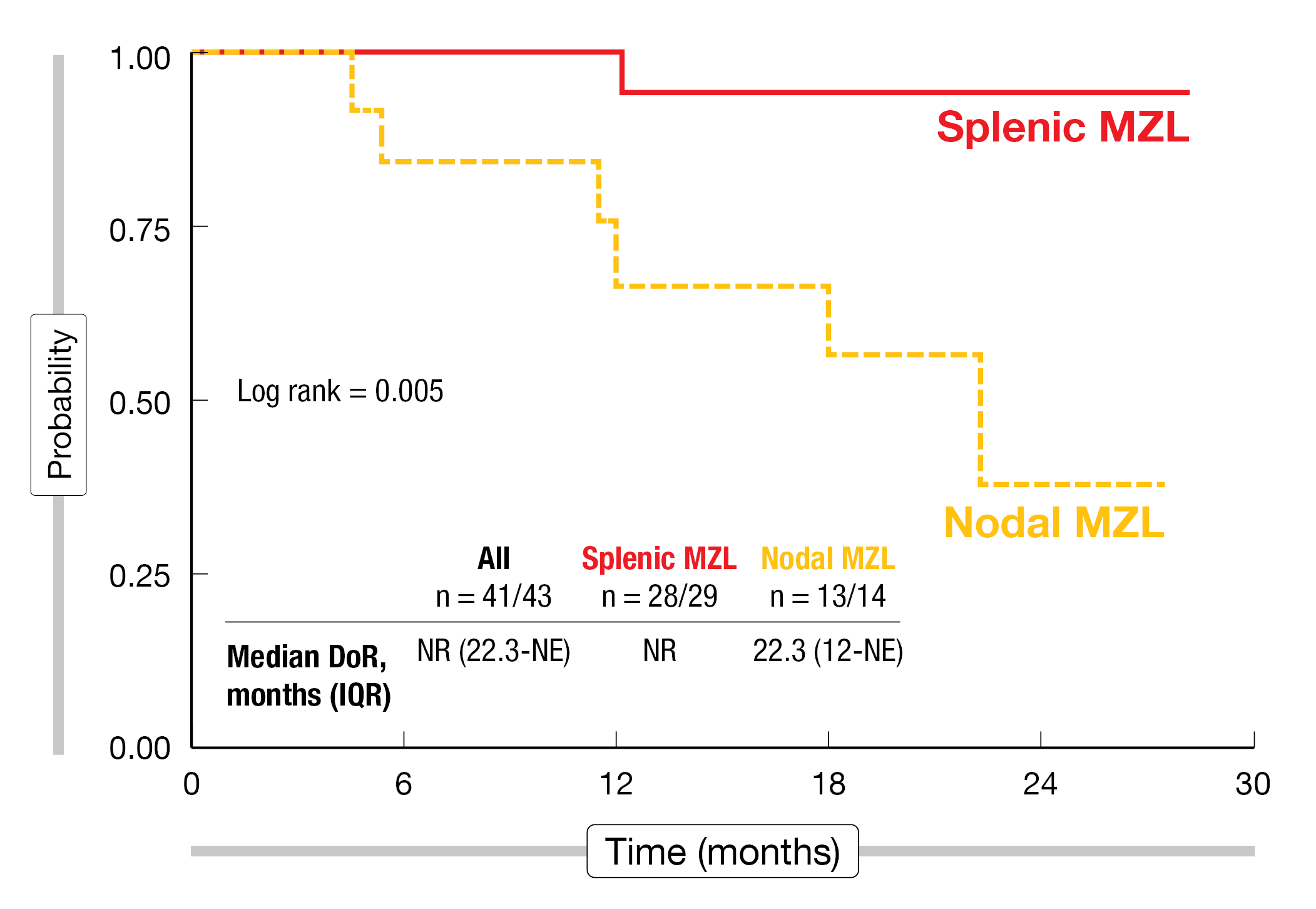
At 12 months, the patients with splenic and nodal MZL showed CR rates of 42 % and 80 %, respectively. In the whole group, this amounted to a CR rate of 53 %. The ORR in the entire cohort was 97 %, with rates of 100 % and 90 % for splenic and nodal MZL, respectively. Best objective response rates did not differ from the remission rates achieved at 12 months. Median duration of response had not been reached in the group with splenic MZL and was 22.3 months for the patients with nodal MZL (Figure 2). Similarly, the PFS results differed greatly across the cohorts. Patients with splenic MZL had a 2-year PFS rate of 86 %, whereas this was 59 % for nodal MZL. OS was identical and excellent in both groups.
The toxicity profile of the combination appeared acceptable, with no obvious differences between splenic and nodal MZL. In all patients, the most common AEs included musculoskeletal pain (all grades, 28.9 %), injection site reactions (28.9 %) and fatigue (17.8 %). Drug-related AEs of specific interest were mainly neutropenia, diarrhea and cardiac events (i.e., hypertension, atrial fibrillation/flutter, ventricular extrasystoles). Two cases of grade ≥ 3 cardiac toxicity were reported. Lower-grade hemorrhage occurred in 3 patients in the overall group, while no grade ≥ 3 events emerged with respect to hemorrhage or infections. Dose reductions were predominantly due to ibrutinib-related AEs such as neutropenia, cardiac disorders, and musculoskeletal pain. In their summary, the authors pointed out that rituximab plus ibrutinib showed promising activity in both splenic and nodal MZL, although some differences were noted in terms of efficacy outcomes.
Figure 2: Duration of response observed with ibrutinib plus rituximab in patients with splenic and nodal marginal zone lymphoma
REFERENCES
- Opat S et al., The MAGNOLIA trial: zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res 2021; 27(23): 6323-6332
- Opat S et al., Long-term efficacy and safety of zanubrutinib in patients with relapsed/refractory marginal zone lymphoma: Final analysis of the MAGNOLIA (BGB-3111-214) trial. EHA 2023, abstract P1084
- Tam CS et al., Rate of atrial fibrillation in patients with B-cell malignancies who undergo treatment with zanubrutinib. 2022 Lymphoma, Leukemia, and Myeloma Congress, abstract 1324736
- Phillips T et al., Zanubrutinib monotherapy in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv 2022; 6(11): 3472-3479
- Noy A et al., Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017; 129(16): 2224-2232
- Noy A et al., Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv 2020; 4(22): 5773-5784
- Özcan M et al., Copanlisib plus rituximab vs placebo plus rituximab in patients with relapsed marginal zone lymphoma treated in the phase III CHRONOS trial. Ann Oncol 2021; 32(Suppl 5): S773-S774
- Thieblemont C et al., Matching-adjusted indirect comparison of zanubrutinib vs ibrutinib in relapsed/refractory marginal zone lymphoma. EHA 2023, abstract P1093
- Thieblemont C et al., Comparative efficacy of zanubrutinib versus rituximab in relapsed marginal zone lymphoma: Matching-adjusted indirect comparison. EHA 2023, abstract P1113
- Thieblemont C et al., Rituximab and ibrutinib combination is safe and effective in untreated splenic and nodal marginal zone lymphomas: planned subset analysis of the IELSG47/MALIBU phase II study. ICML 2023, abstract 65
© 2023 Springer-Verlag GmbH, Impressum
More posts
DLBCL: treatment of elderly patients and relapsed disease
DLBCL: treatment of elderly patients and relapsed disease POLAR BEAR: novel regimen fr
Waldenström macroglobulinemia: findings from ASPEN and BRUIN
Waldenström macroglobulinemia: findings from ASPEN and BRUIN ASPEN: HRQoL for zanubrut
Outcome improvements in relapsed and untreated marginal zone lymphoma
Outcome improvements in relapsed and untreated marginal zone lymphoma Final analysis o
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors Mosun
Current insights into BTK inhibition and other targeted approaches in CLL
Current insights into BTK inhibition and other targeted approaches in CLL Treatment-na
Preface – ASCO/EHA/ICML 2023
Preface – ASCO/EHA/ICML 2023 © Dirk Gillissen – Arnon P. Kater, MD, PhD, Department o