Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Mosunetuzumab: CR at EOT
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL) subtype [1]. In general, it remains incurable with standard therapies, and most patients experience multiple relapses over time. Therefore, there remains a great need for innovative new regimens such as the first-in-class CD20xCD3 T-cell–engaging bispecific antibody mosunetuzumab that is being tested in a pivotal phase II trial in patients with relapsed/refractory grade 1-3a FL after ≥ 2 lines of therapy (including an anti-CD20 antibody and an alkylating agent). Mosunetuzumab Q3W is administered intravenously for a fixed period: patients achieving complete remission (CR) after 8 cycles discontinue their treatment at that time, while those with partial remission or disease stabilization can receive another 9 cycles for a total of 17 cycles. Indeed, mosunetuzumab has been shown to induce a high CR rate and durable responses [2]. Cytokine release syndrome (CRS) was reported as the most common AE, although this was predominantly grade 1 or 2.
At ICML 2023, Sehn et al. presented an update after a median follow-up of 28.3 months for 90 patients, with a focus on individuals who achieved CR by the end of treatment (EOT) [3]. Most patients (82 %) received 8 or fewer cycles. Fifty-four (60 %) obtained CR as best response; at EOT, CR was present in 49 individuals (54 %). Among the remaining five patients, one with CR developed disease progression in cycle 8, while the other four achieved CR only after EOT due to delayed bone marrow confirmation.
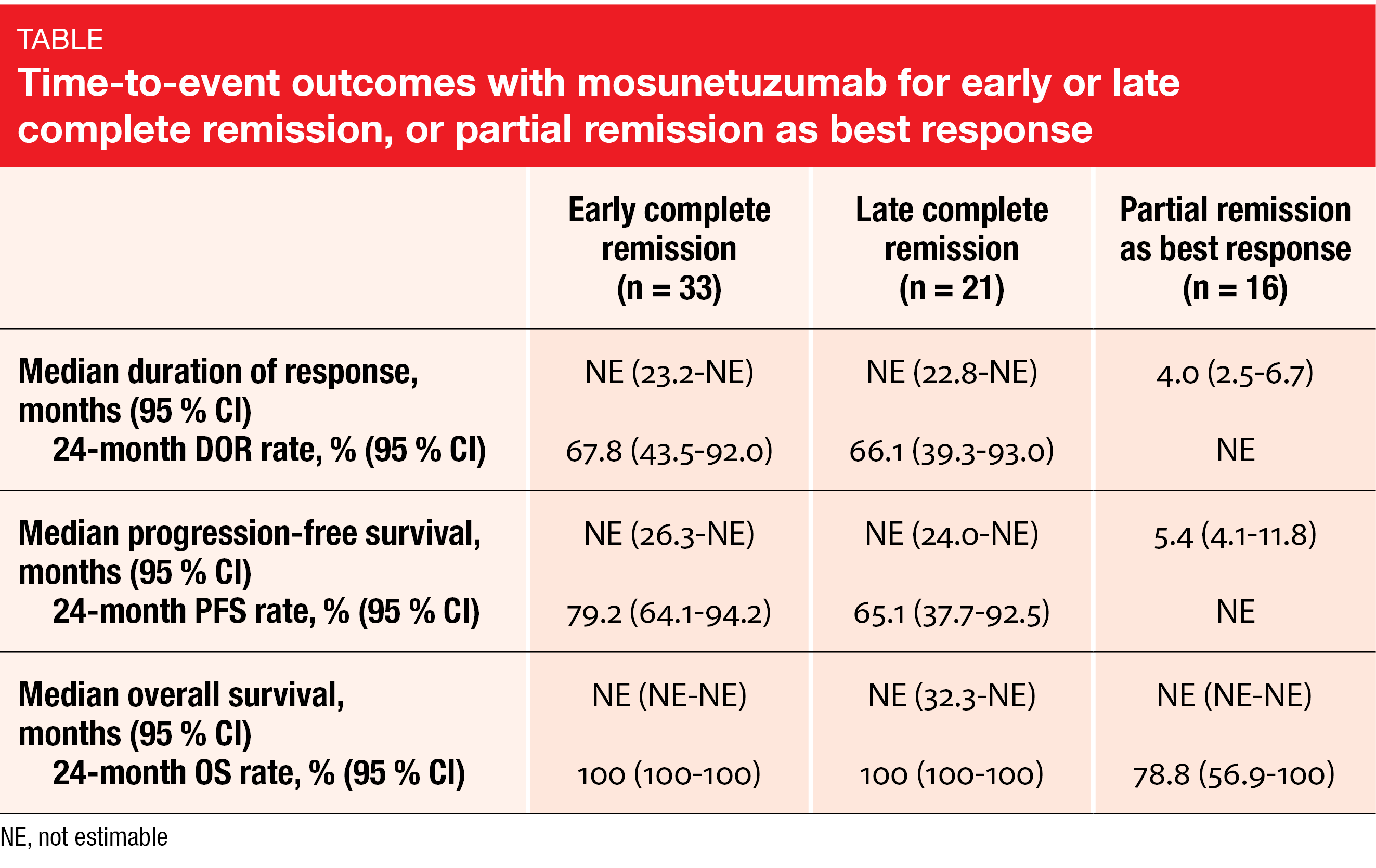
The 49 patients in a confirmed CR at the EOT had durable responses. Median duration of response (DOR) had not been reached yet in this group at data cutoff. At 24 months, 100 % of patients were alive, 65 % retained CR, and 77 % were progression-free. The time to first CR did not affect the outcomes, as patients with early CR (by month 3) and late CR (after month 3) showed similar results for DOR, progression-free survival (PFS), and overall survival (OS; Table). In contrast, patients with partial remission as best response fared considerably worse regarding these endpoints.
An exploratory analysis revealed no correlation between total metabolic tumor volume (TMTV) at baseline and best overall response. Importantly, many patients who achieved CR had a high TMTV at baseline. Moreover, the researchers sought to determine factors conferring an increased likelihood of grade ≥ 2 CRS. These data suggested more frequent grade ≥ 2 CRS rates in patients with bone or bone marrow disease at baseline compared to those without (33.3 % vs. 13.8 %).
First pivotal results for odronextamab
Novelli et al. reported first interim results from the open-label, pivotal phase II ELM-2 study investigating the CD20xCD3 bispecific antibody odronextamab [4]. ELM-2 contains five disease-specific cohorts for different types of relapsed/refractory B-NHL. The FL cohort includes patients with grade 1-3a FL who are refractory to or relapsed after ≥ 2 prior lines of therapy including an anti-CD20 antibody and an alkylator. Odronextamab 160 mg is administered intravenously Q2W after step-up dosing in cycle 1 and 3 doses on days 1, 8 and 15 in cycles 2–4, with 21 days per cycle. The step-up regimen had to be optimized in the course of the study to further mitigate the CRS risk; approximately half of the study population received the 1/20/80 mg regimen, while the other half was treated with the 0.7/4/20 mg regimen. The latter included 0.7 mg on days 1/2, 4 mg on days 8/9, and 20 mg on days 15/16 of cycle 1. In cycles 2–4, 80 mg were administered on days 1, 8 and 15. Objective response rate (ORR) by independent central review is defined as the primary endpoint.
In this heavily pretreated population (n = 131), 30.5 % of patients had previously undergone autologous stem cell transplantation, 43.5 % were double refractory to an anti-CD20 antibody and an alkylator, and almost half had experienced progression within 24 months of starting first-line treatment (POD24). In spite of these high-risk characteristics, the ORR was 81.8 %, with 75.2 % of patients achieving CR. High response rates were observed across high-risk subgroups; also, ORR and CR rates were similar regardless of the type of step-up regimen administered in cycle 1. Also, responses appeared durable, with median duration of CR of 20.5 months and a median PFS of 20.2 months. Median OS had not been reached yet at the time of the analysis. The 18-month PFS and OS rates were 55.3 % and 76.3 %, respectively.
With the optimized step-up regimen, odronextamab had a generally manageable safety profile. CRS events as the most common treatment-related AEs occurred in 55.7 % but were predominantly low-grade and mainly emerged during cycle 1. No immune effector cell-associated neurotoxicity syndrome or tumor lysis syndrome emerged in the group treated with the optimized step-up strategy. Three treatment-related grade 5 AEs were reported. The incidence of AEs leading to treatment discontinuation was low at 7.6 %.
In their summary, the authors pointed out that these first results from the pivotal phase II trial of odronextamab demonstrate a new benchmark for efficacy in heavily pretreated, relapsed/refractory FL. Randomized, controlled phase III studies will be initiated in earlier lines of treatment.
Epcoritamab according to POD24 status
The CD3xCD20 bispecific antibody epcoritamab, which is administered subcutaneously, is being assessed in addition to rituximab and lenalidomide (R2) in the ongoing phase Ib/2 EPCORE NHL-2 trial. Patients with relapsed/refractory CD20-positive FL grade 1-3a and stage II-IV disease are receiving two different schedules of epcoritamab 48 mg (arms 2a and 2b) for up to 2 years in combination with R2 in cycles 1–12. The first pooled analysis of arms 2a and 2b was presented at the ASCO 2023 Congress by Merryman et al. [5]. Many of the 111 enrolled patients had high-risk features, such as POD24 (n = 42), primary refractory disease (n = 38), refractoriness to prior anti-CD20 therapy (n = 48), double refractory disease (n = 37), and FLIPI 3–5 (n = 64). Only 21 patients did not fall into any of these categories. In patients experiencing POD24, no standard of care has been established to date, as current therapeutic options provide suboptimal clinical results [6-8].
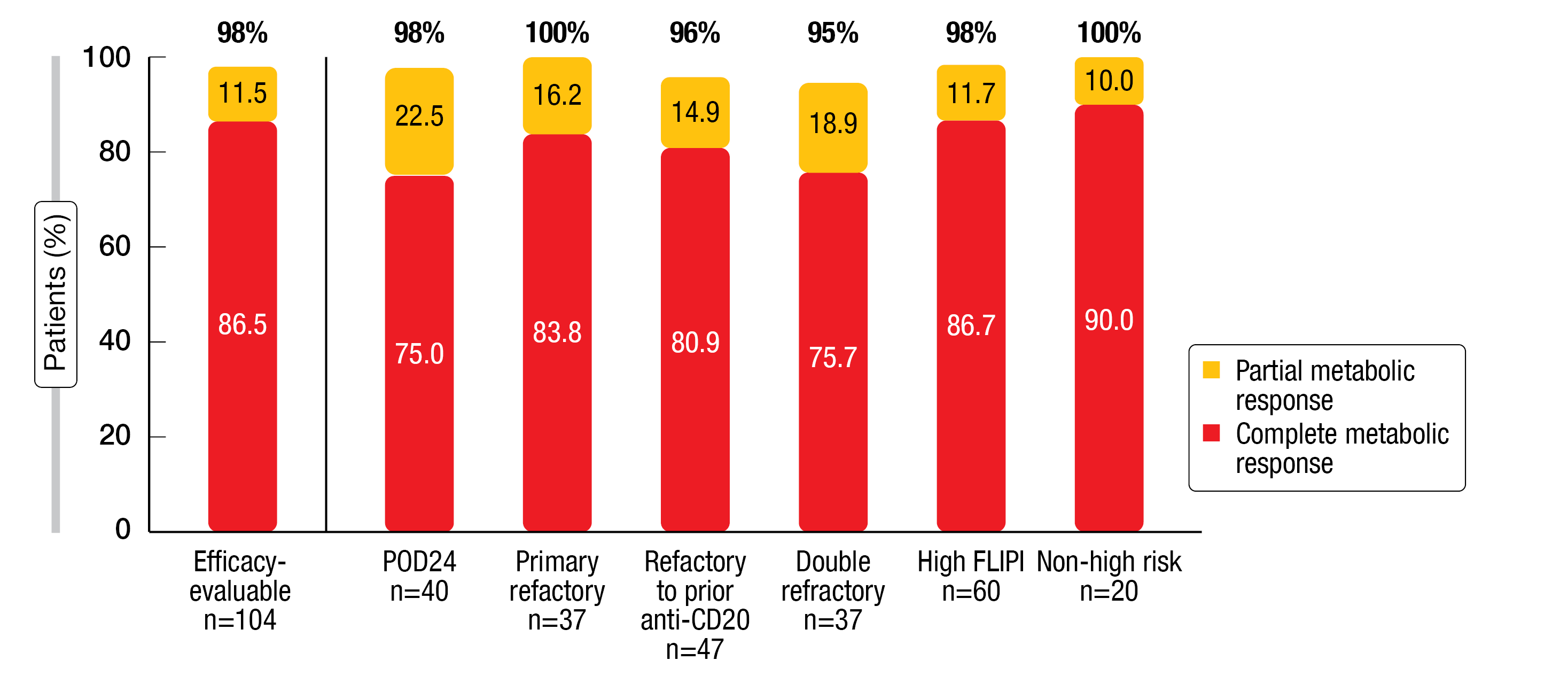
Within the efficacy-evaluable cohort (n = 104), the ORR was 98 %, and 87 % of patients achieved a complete metabolic response (CMR). These response rates were higher than the ORR and CMR obtained with the immediate prior line of therapy (85 % and 58 %, respectively). Moreover, the analysis revealed consistently high CMR and partial metabolic response rates across all high-risk subgroups including those with POD24 (Figure 1). Median duration of CR and median PFS had not been reached in the overall and POD24 populations.
Epcoritamab plus R2 displayed a manageable safety profile that was consistent with previous reports. CRS events occurred in a predictable manner, were primarily low-grade, and all of them resolved. No clinical tumor lysis syndrome events were observed. Combination regimens containing epcoritamab are being assessed in the randomized, phase III EPCORE FL-1 study (NCT05409066) and in a POD24 cohort in the EPCORE NHL-2 trial (NCT04663347).
Figure 1: Complete and partial metabolic responses with epcoritamab plus R2 in various subgroups
ROSEWOOD: zanubrutinib + obinutuzumab
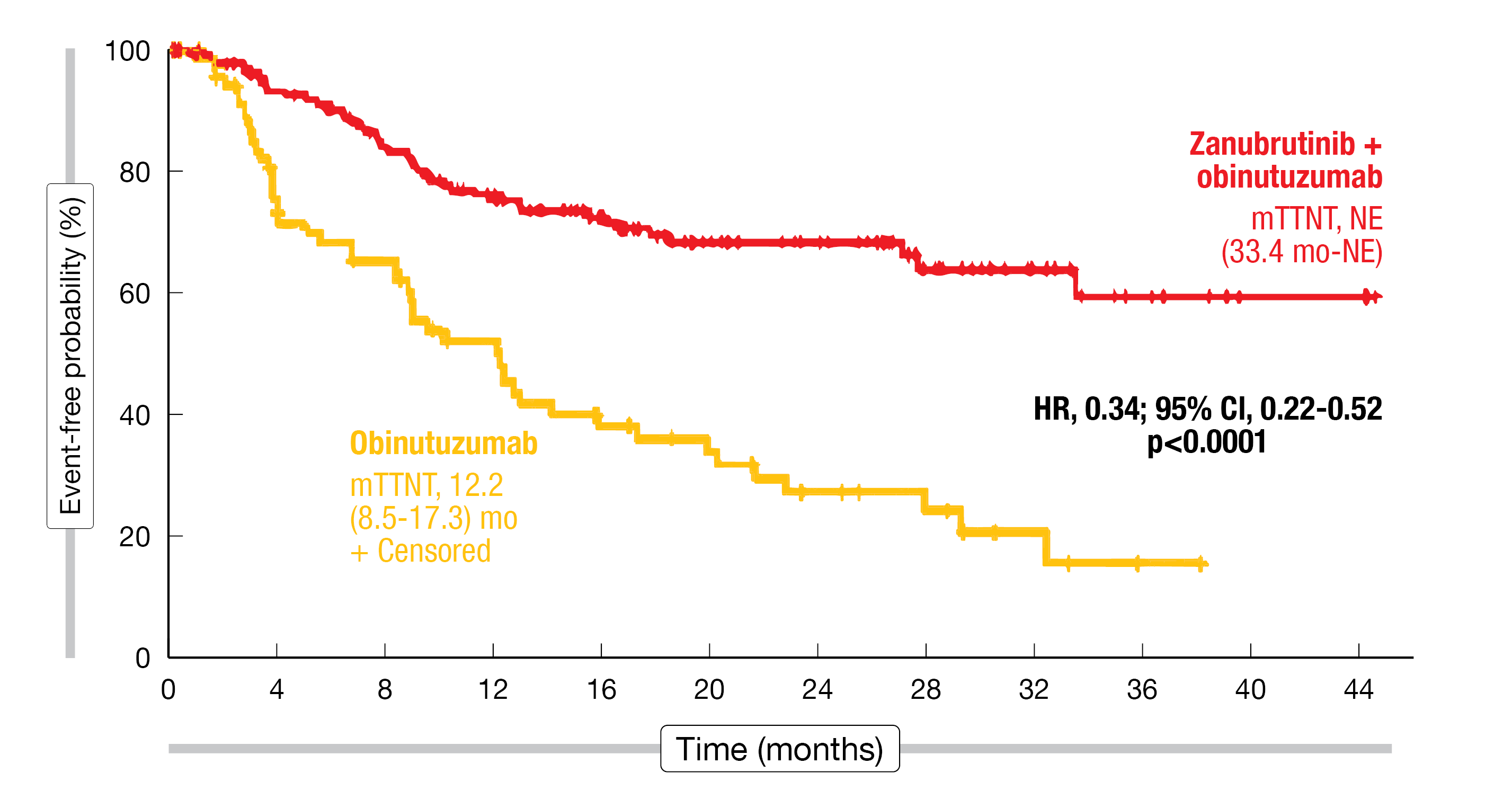
The second-generation BTK inhibitor zanubrutinib is being tested in the randomized phase II ROSEWOOD trial. Patients with grade 1-3a relapsed/refractory FL after ≥ 2 lines of treatment including an anti-CD20 antibody and an alkylating agent were randomized to receive zanubrutinib plus obinutuzumab (n = 145) versus single-agent obinutuzumab (n = 72) until disease progression. The trial previously met its primary endpoint of ORR by independent review (68.3 % vs. 45.8 %; p = 0.0017) [9]. Updated findings presented at ICML 2023 after a median follow-up of 20.2 months showed significant benefits of the combined treatment in terms of ORR (69.0 % vs. 45.8 %; p = 0.0012) and CR (39.3 % vs.19.4 %; p = 0.0035) [10]. Median DOR had not been reached in the experimental arm and was 14.0 months in the control arm. Likewise, median duration of CR had not been reached and was 26.5 months, respectively. At 18 months, 69.3 % vs. 41.9 % of patients had responded, and 87.4 % vs. 51.1 % had a CR. The combination showed consistent ORR benefit across all prespecified subgroups. Furthermore, significant superiority of zanubrutinib plus obinutuzumab was demonstrated for PFS (28.0 vs. 10.4 months; HR, 0.50; p = 0.0007) and time to next anti-lymphoma treatment (not reached vs. 12.2 months; HR, 0.34; p < 0.0001; Figure 2). Regarding OS, the analysis demonstrated a trend favoring the combination (not reached vs. 34.6 months; HR, 0.62; p = 0.0845).
No unexpected safety findings emerged with the prolonged follow-up. Diarrhea and fatigue were the most common AEs in the experimental arm, while pyrexia and infusion-related reactions occurred more frequently with obinutuzumab alone. Among grade ≥ 3 non-hematologic treatment-emergent AEs, pneumonia was reported most commonly (9.8 % vs. 4.2 %). Median treatment exposure for zanubrutinib plus obinutuzumab was twice that for obinutuzumab monotherapy (12.2 vs. 6.5 months). After adjustment for exposure, the incidence rates of treatment-emergent AEs of special interest were similar for both arms with the exception of any-grade hemorrhage (2.4 vs. 1.3 persons/100 person-months).
According to the authors, zanubrutinib plus obinutuzumab might represent a potential novel combination therapy for patients with relapsed/refractory FL. The global, randomized, open-label, phase III MAHOGANY study is currently investigating zanubrutinib plus obinutuzumab versus lenalidomide plus rituximab in patients with relapsed/refractory grade 1-3a FL after ≥ 1 prior treatment line that included an anti-CD20 agent (NCT05100862) [11]. MAHOGANY contains two independent cohorts for patients with relapsed/refractory FL and marginal zone lymphoma.
Figure 2: Time to next anti-lymphoma treatment for zanubrutinib plus obinutuzumab vs. obinutuzumab alone
First-line rituximab with or without ibrutinib
The randomized, double-blind phase II SAKK35/14 trial explored chemotherapy-free frontline treatment with ibrutinib 560 mg OD (n = 94) or placebo (n = 98) for 24 months in addition to 16 administrations of rituximab in patients with advanced FL who required therapy. Need of treatment was defined as ≥ 1 of several criteria including B symptoms, clinically significant progression over ≥ 6 months, and bulky disease ≥ 6 cm in long diameter, among others.
CR at 24 months according to independent review constituted the primary objective. After a median follow-up of 42 months, this endpoint was not met (40 % vs. 36 %; OR, 0.80; p = 0.233) [12]. However, ibrutinib plus rituximab performed significantly better than rituximab alone with regard to the ORR rate at 24 weeks (73 % vs. 59 %; p = 0.046). In the ibrutinib arm, there were trends toward improved PFS (3-year rates, 45 % vs. 37 %; p = 0.056) and time to next treatment (not reached vs. 41.2 months; p = 0.099). At 3 years, 96 % of patients were alive in both arms.
As expected, the combined regimen conferred increased toxicity. Among grade ≥ 3 AEs, neutropenia (14 % vs. 8 %), hypertension (5 % each) and skin rash (10 % vs. 5 %) were most common. According to the authors, the improvements in secondary endpoints observed with ibrutinib plus rituximab indicate a potential clinical benefit that could be further explored. Moreover, the very
good OS in both arms supports the current use of rituximab as a therapeutic partner in clinical trials of novel combinations.
REFERENCES
- Freedman A, Jacobsen E, Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol 2020; 95(3): 316-327
- Budde LE et al., Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol 2022; 23(8): 1055-1065
- Sehn LH et al., Mosunetuzumab demonstrates durable responses in patients with relapsed and/or refractory follicular lymphoma who have received ≥ 2 prior therapies: updated analysis of a pivotal phase II study. ICML 2023, abstract 83
- Novelli S et al., Odronextamab in patients with relapsed/refractory follicular lymphoma grade 1-3a: results from a prespecified analysis of the pivotal phase 2 study ELM-2. ICML 2023, abstract 82
- Merryman RW et al., Epcoritamab + R2 regimen and responses in high-risk follicular lymphoma, regardless of POD24 status. J Clin Oncol 41, 2023 (suppl 16; abstr 7506)
- Casulo C et al., Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare Study. J Clin Oncol 2015; 33(23): 2516-2522
- Casulo C et al., Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood 2022; 139(11): 1684-1693
- Andorsky DJ et al., Phase IIIb randomized study of lenalidomide plus rituximab (R2) followed by maintenance in relapsed/refractory NHL: analysis of patients with double-refractory or early relapsed follicular lymphoma (FL). J Clin Oncol 2017; 35(suppl), abstract 7502
- Zinzani PL et al., Zanubrutinib plus obinutuzumab versus obinutuzumab monotherapy in patients with relapsed or refractory follicular lymphoma: primary analysis of the phase 2 randomized ROSEWOOD trial. J Clin Oncol 40, 2022 (suppl 16; abstr 7510)
- Zinzani PL et al., Zanubrutinib plus obinutuzumab versus obinutuzumab in patients with relapsed/refractory follicular lymphoma: Updated analysis of the ROSEWOOD study. ICML 2023, abstract 81
- Sehn LH et al., MAHOGANY: A phase 3 trial of zanubrutinib plus anti-CD20 versus lenalidomide plus rituximab in patients with relapsed/refractory follicular or marginal zone lymphoma. ICML 2023, abstract OT4
- Østenstad B et al., SAKK35/14 randomized trial of rituximab with or without ibrutinib for untreated patients with advanced follicular lymphoma in need of therapy. ICML 2023, abstract 80
© 2023 Springer-Verlag GmbH, Impressum
More posts
DLBCL: treatment of elderly patients and relapsed disease
DLBCL: treatment of elderly patients and relapsed disease POLAR BEAR: novel regimen fr
Waldenström macroglobulinemia: findings from ASPEN and BRUIN
Waldenström macroglobulinemia: findings from ASPEN and BRUIN ASPEN: HRQoL for zanubrut
Outcome improvements in relapsed and untreated marginal zone lymphoma
Outcome improvements in relapsed and untreated marginal zone lymphoma Final analysis o
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors Mosun
Current insights into BTK inhibition and other targeted approaches in CLL
Current insights into BTK inhibition and other targeted approaches in CLL Treatment-na
Preface – ASCO/EHA/ICML 2023
Preface – ASCO/EHA/ICML 2023 © Dirk Gillissen – Arnon P. Kater, MD, PhD, Department o