DLBCL: treatment of elderly patients and relapsed disease
POLAR BEAR: novel regimen from the age of 75
Overall survival has improved considerably in the setting of diffuse large B-cell lymphoma (DLBCL). However, patients above the age of 80 years are an exception in terms of survival prolongation and therefore face an unmet clinical need [1]. At the same time, this is a group that constitutes an increasing proportion of DLBCL patients. The current treatment standard in elderly patients with DLBCL is rituximab plus miniCHOP (R-miniCHOP) [2]. Innovative approaches include the combination of rituximab with the antibody-drug conjugate polatuzumab vedotin and the modified chemotherapy regimen miniCHP that is administered without vincristine (R-pola-miniCHP). The ongoing randomized phase III POLAR BEAR trial is comparing 6 courses of R-pola-miniCHP with 6 courses of R-miniCHOP in patients aged ≥ 80 years or frail patients aged 75-80 years with DLBCL, which also includes transformation from indolent lymphoma, or other types of high-risk B-cell lymphoma. Progression-free survival (PFS) has been defined as the primary endpoint.
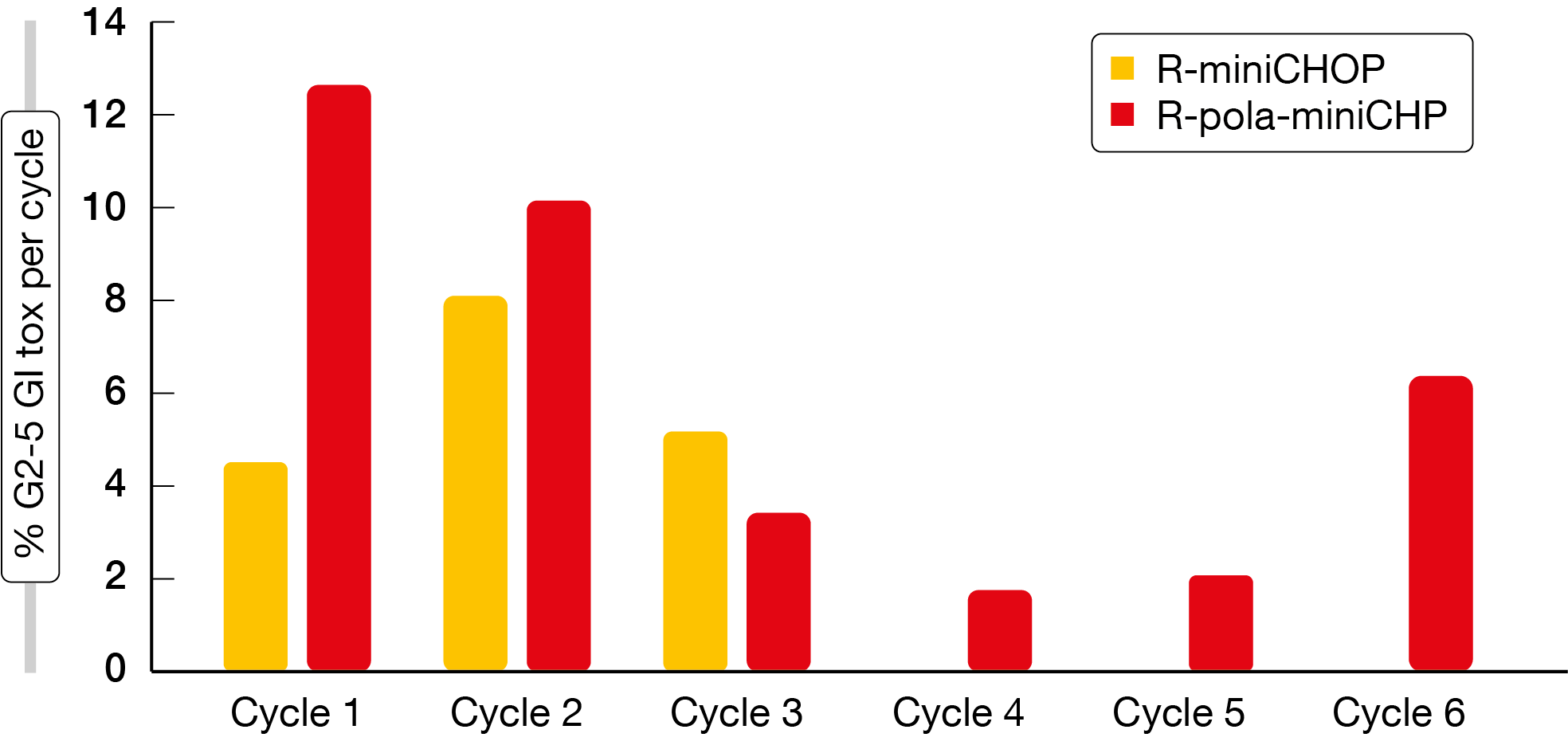
The safety data presented at EHA 2023 related to 140 patients the majority of whom had DLBCL (87.5 % and 92.4 % in the R-pola-miniCHP and R-miniCHOP arms, respectively) [3]. Adverse events (AEs) in both arms were mainly categorized as “other”, as these could not be classified further; here, grade 1 and 2 events occurred more commonly with R-pola-miniCHP than with R-miniCHOP. Infections and gastrointestinal AEs ranked second and third, respectively. The majority of all AEs were grade 1 or 2, and the safety profiles were largely comparable across the two study arms. Grade ≥ 3 anemia was more common in the experimental arm (14 % vs. 2.8 %), as was gastrointestinal toxicity of all grades (55 % vs. 31 %) and of grade ≥ 3 (30 % vs. 17 %). Infections occurred somewhat more frequently with R-pola-miniCHP (57 % vs. 45 %), while the grade ≥ 3 infection rates did not differ across the arms (16 % vs. 14 %). In contrast, cardiovascular AEs were observed more commonly with the comparator regimen (23 % vs. 30 %). The rates for grade 1 peripheral neuropathy were similar for both regimens (13 % vs. 9.9 %). Gastrointestinal toxicity mostly consisted of diarrhea, which predominantly occurred early on during the treatment (Figure 1).
After a median follow-up of 1.1 years, median PFS had not been reached in the pooled population. At 2 years, the PFS rate was 65 %. Further analyses assessed potential associations between PFS and age (< 80 years, 80-85 years, > 85 years) as well as the degree of comorbidity (CIRS-G < 10, CIRS-G ≥ 10) but found no impact of these factors on PFS. The authors concluded that both R-pola-miniCHP and R-miniCHOP are tolerable in elderly patients with DLBCL, with encouraging early pooled efficacy data for the novel combination.
Figure 1: Grade 2-5 gastrointestinal toxicity in the POLAR BEAR trial
Odronextamab in the relapsed/refractory setting
Approximately one third of DLBCL patients ultimately develop relapsed/refractory disease, which remains a major cause of mortality [4]. This raises a significant unmet need for effective off-the-shelf therapies, especially in patients who have no access to or are ineligible for treatment options such as CAR T-cell therapy or high-dose therapy/stem cell transplantation. The CD20xCD3 bispecific antibody odronextamab is investigated as single agent in patients with relapsed/refractory B-cell non-Hodgkin lymphoma in the pivotal open-label, multicohort, phase II ELM-2 trial. First interim results for the DLBCL cohort (n = 140) were reported at EHA 2023 by Walewski et al. [5].
The patients have received ≥ 2 lines of pretreatment including an anti-CD20 antibody and an alkylator. In 90.7 %, the disease is refractory to any prior line of therapy, and 65.7 % of patients show double refractoriness to an alkylator/anti-CD20 antibody treatment in any line. Odronextamab is administered i. v. with step-up in cycle 1 followed by 160 mg on days 1, 8 and 15 in cycles 2–4 and 320 mg Q2W from cycle 5 onwards until progression. During the study, the step-up in cycle 1 was modified from a 1/20 mg regimen to a 0.7/4/20 mg regimen to further mitigate the risk of cytokine release syndrome (CRS). The objective response rate (ORR) by independent central review is defined as the primary endpoint.
Clinically relevant antitumor activity of odronextamab was observed in this heavily pretreated, highly refractory population. The ORR was 49.2 %, and complete responses (CRs) occurred in 30.8 %. Responses were durable, with an 18-month PFS rate of 26.0 % and median duration of CR of 17.9 months. Consistent ORR and CR rates resulted at 12 weeks irrespective of the type of step-up regimen in cycle 1. Patients with prior CAR T-cell therapy who had enrolled in the phase I dose expansion cohort were analyzed separately (n = 31). Similar to the overall population, they showed ORR and CR rates of 48.4 % and 32.3 %, respectively. Median duration of CR had not been reached in this group at the time of the analysis.
Odronextamab generally exhibited a manageable safety profile with the optimized 0.7/4/20 mg step-up regimen that reduced the rate of grade 3 CRS events to 1.4 %. Approximately half of patients developed CRS that was mostly grade 1. No grade 4 or 5 CRS events were noted. Moreover, no cases of tumor lysis syndrome, infusion-related reactions or grade ≥ 3 immune effector cell-associated neurotoxicity syndrome emerged with the optimized step-up treatment. Any-grade infections were reported in 58.9 %; the majority of these were grade 1 or 2 (32.9 %), although grade 5 events occurred in 9.6 %. Discontinuation was due to treatment-related AEs in 7.9 %. Randomized controlled phase III trials will be initiated to further investigate odronextamab in earlier lines of treatment.
Zanubrutinib plus lenalidomide: phase I data
The BTK inhibitor zanubrutinib in addition to lenalidomide is tested in Chinese patients with relapsed/refractory DLBCL after ≥ 1 prior treatment line and ineligibility for stem cell transplantation (if not received previously) in the ongoing open-label phase I BGB-3111-110 study. Zanubrutinib 160 mg BID and lenalidomide 25 mg OD on days 1-21 per 28-day cycle were identified as the recommended part 2 dose (RP2D) in the dose escalation part [6]. At ASCO 2023, Zhang et al. presented the results of the interim analysis for a total of 46 patients included in the dose escalation and dose expansion parts of the study [7]. Thirty of them had received the RP2D. ORR constitutes the primary endpoint of the dose expansion phase.
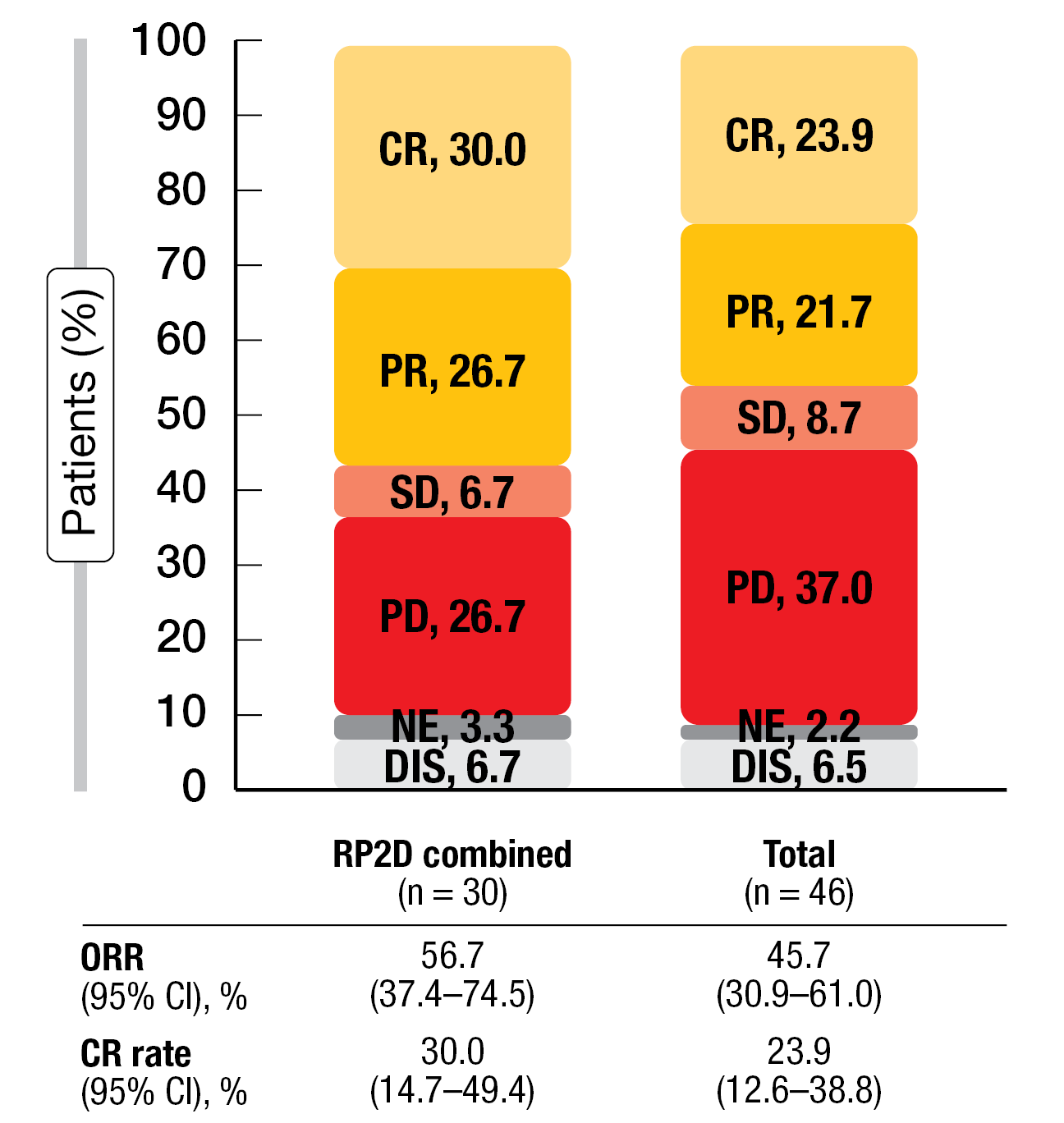
The ORR was 45.7 % overall, with CRs observed in 23.9 %; at the RP2D, these rates were 56.7 % and 30.0 %, respectively (Figure 2). Patients with the non-GCB subtype experienced higher CR rates than those with the GCB subtype in both the total group (28.1 % and 16.7 %, respectively) and the RP2D cohort (34.8 % and 16.7 %, respectively). The ORRs ranged from 46.9 % to 60.9 % across these groups. In the overall population, median duration of response had not been reached yet, and the 6-month event-free rate was 63.5 %. Median PFS was 5.5 months, with a 9-month event-free rate of 31.1 %.
Grade ≥ 3 treatment-emergent AEs occurred in 60.9 % of patients. Most commonly, they consisted of hematologic toxicities such as neutrophil count decreases that were generally manageable across all dose levels. In the RP2D group, one patient (3.3 %) had febrile neutropenia but recovered within 2 days. Treatment-emergent AEs that prompted discontinuation were observed in 8.7 %. No fatal treatment-related events occurred. In their conclusion, the authors noted that zanubrutinib plus lenalidomide demonstrated an acceptable safety profile and promising efficacy. Further evaluation of this combination in a larger sample is planned.
Figure 2: Responses to zanubrutinib plus lenalidomide in the RP2D and total cohorts
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; DIS, discontinued prior to first treatment; RP2D, recommended part 2 dose
REFERENCES
- Székely E et al., Improvement in survival of diffuse large B-cell lymphoma in relation to age, gender, International Prognostic Index and extranodal presentation: a population based Swedish Lymphoma Registry study. Leuk Lymphoma 2014; 55(8): 1838-1843
- Peyrade F et al., Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011; 12(5): 460-468
- Jerkeman M et al., Initial safety data from the phase 3 POLAR BEAR trial in elderly or frail patients with diffuse large cell lymphoma. EHA 2023, abstract S227
- Poletto S et al., Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev 2022; 110: 102443
- Walewski J et al., Odronextamab in patients with relapsed/refractory diffuse large B-cell lymphoma: results from a prespecified analysis of the pivotal phase 2 study ELM-2. EHA 2023, abstract P1115
- Zhang H et al., Preliminary safety and efficacy of zanubrutinib in combination with lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma. ASH 2022, abstract 1627
- Zhang H et al., First interim analysis of a phase 1 study of zanubrutinib plus lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma. J Clin Oncol 41, 2023 (suppl 16; abstr 7557)
© 2023 Springer-Verlag GmbH, Impressum
More posts
DLBCL: treatment of elderly patients and relapsed disease
DLBCL: treatment of elderly patients and relapsed disease POLAR BEAR: novel regimen fr
Waldenström macroglobulinemia: findings from ASPEN and BRUIN
Waldenström macroglobulinemia: findings from ASPEN and BRUIN ASPEN: HRQoL for zanubrut
Outcome improvements in relapsed and untreated marginal zone lymphoma
Outcome improvements in relapsed and untreated marginal zone lymphoma Final analysis o
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors Mosun
Current insights into BTK inhibition and other targeted approaches in CLL
Current insights into BTK inhibition and other targeted approaches in CLL Treatment-na
Preface – ASCO/EHA/ICML 2023
Preface – ASCO/EHA/ICML 2023 © Dirk Gillissen – Arnon P. Kater, MD, PhD, Department o