Updates on BTK- and Bcl-2–targeted treatment in various B-cell malignancies
Ibrutinib plus CIT: SELENE trial
Survival outcomes typically deteriorate with repeated lines of chemoimmunotherapy (CIT) in patients with relapsed/refractory non-Hodgkin lymphoma. The randomized, double-blind phase III SELENE trial was conducted to determine whether the addition of the BTK inhibitor ibrutinib to CIT in patients with relapsed/refractory follicular lymphoma (FL) or marginal zone lymphoma (MZL) improves progression-free survival (PFS). CIT consisted of either bendamustine/rituximab (BR) or R-CHOP. In the control arm, placebo was administered in addition to these regimens. Each arm contained 200 patients pretreated with ≥ 1 anti-CD20 CIT strategy. The majority had FL (86.1 % in both arms), and 90 % received BR as the backbone CIT. Nastoupil et al. presented the results of SELENE at ICML 2023 [1].
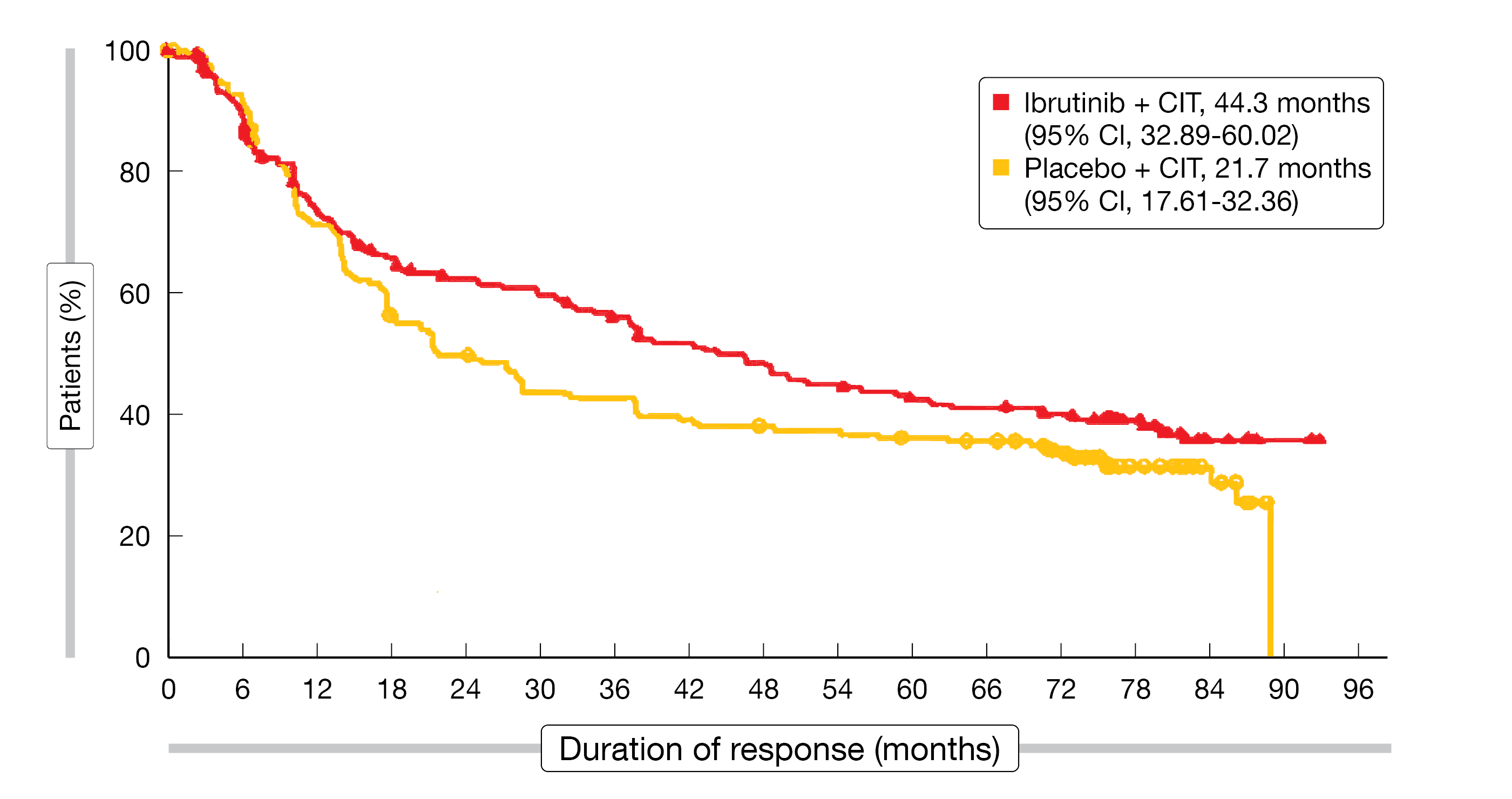
Ibrutinib plus CIT did improve PFS compared to CIT alone, although the primary study endpoint was not met (40.5 vs. 23.8 months; HR, 0.81; p = 0.0922). In each arm, 28 patients had MZL; this group fared better with both regimens than the overall population. Median PFS by investigator had not been reached with ibrutinib plus CIT and was 91.6 months with placebo plus CIT in the MZL cohort (HR, 0.52; p = 0.1118). The overall response rates (ORRs) did not differ across the study arms in the ITT population (91.6 % vs. 90.5 %), with complete responses observed in 55.0 % vs. 50.2 %. Similar ORRs and complete response rates were found in the MZL subgroup. Median duration of response in the overall population was longer with ibrutinib plus CIT than with CIT alone (44.3 vs. 21.7 months; Figure 1). Median OS had not been reached with either treatment. At 7 years, the OS rates did not differ across the arms (67.4 % vs. 68.3 %), with similar rates for the MZL cohort (76.6 % vs. 68.5 %; HR, 0.69; p = 0.4964).
The overall safety profile of ibrutinib plus CIT was consistent with the known profiles of the individual agents. Grade ≥ 3 thrombocytopenia and anemia occurred more frequently with the ibrutinib-based therapy than with CIT alone (10.0 % vs. 5.0 % and 11.4 % vs. 4.0 %, respectively). Treatment-emergent AEs (TEAEs) leading to dose reduction were more common in the experimental arm (20.4 % vs. 10.6 %), as were TEAEs leading to discontinuation (30.8 % vs. 18.6 %). The authors concluded that the administration of ibrutinib resulted in additive toxicity but did not impact OS. Further analyses are required to define specific FL/MZL subgroups that might benefit from extended treatment with ibrutinib following CIT.
Figure 1: Duration of response with ibrutinib vs. placebo plus chemoimmunotherapy
Zanubrutinib: pooled safety data
Continuous therapy with ibrutinib and acalabrutinib is effective in B-cell malignancies, although many patients discontinue treatment with these BTK inhibitors due to intolerance potentially caused by off-target kinase binding [2]. The next-generation BTK inhibitor zanubrutinib has been designed to maximize tolerability by minimizing off-target kinase binding [3]. Pooled safety analyses reported at ICML 2023 showed that zanubrutinib is well tolerated in patients with B-cell malignancies including chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), MZL, Waldenström macroglobulinemia (WM), FL, and other entities [4]. Data from 1,550 patients treated with zanubrutinib und 422 treated with ibrutinib were included.
Zanubrutinib-related TEAEs were generally mild to moderate; the most common grade ≥ 3 TEAEs were pneumonia (8.4 %) and hypertension (8.1 %). Compared with ibrutinib, exposure-adjusted incidence rates of AEs of special interest were numerically lower in head-to-head comparisons of the ASPEN/ALPINE study populations, with the exception of neutropenia. Exposure-adjusted incidence rates were significantly lower with zanubrutinib for atrial fibrillation and infections (p < 0.0001 and p = 0.0098, respectively). Hypertension tended to increase over time with ibrutinib, whereas it remained relatively stable with zanubrutinib. The prevalence of atrial fibrillation remained lower with zanubrutinib than with ibrutinib. Mortality due to cardiac events was lower with zanubrutinib (0.2 % vs. 1.7 %). Overall, these analyses support zanubrutinib as an appropriate long-term treatment option for patients with B-cell malignancies.
Improved tolerability after BTKi and in the real world
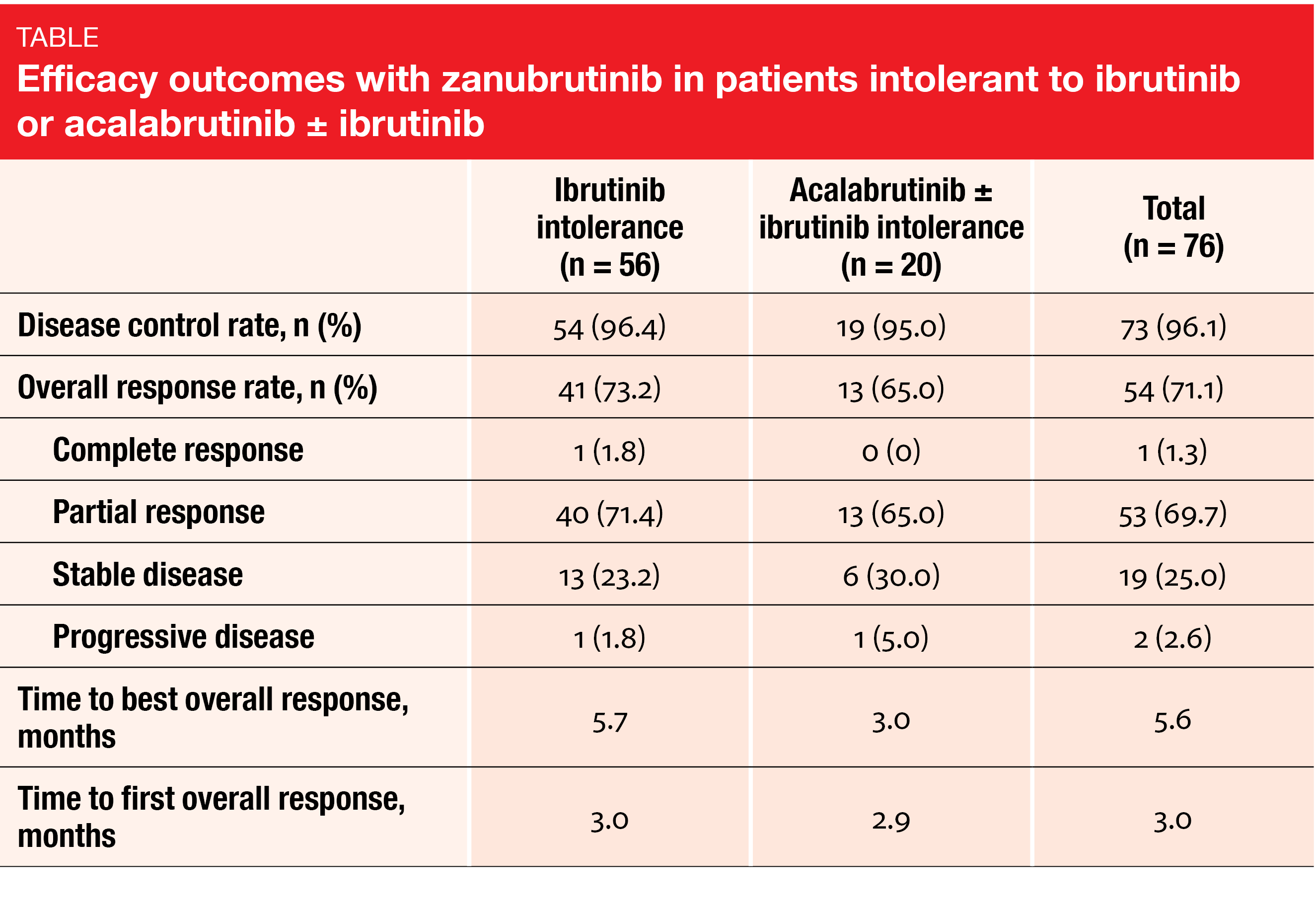
Updated results from the phase II BGB-3111-215 study that investigated zanubrutinib in patients with CLL/SLL, MCL, MZL or WM who were intolerant of ibrutinib (n = 57) or acalabrutinib ± ibrutinib (n =25) were reported by Shadman et al. at a median follow-up of 25.2 months [5]. According to this analysis, 67.7 % of ibrutinib-intolerance events and 73.0 % of acalabrutinib-intolerance events did not recur on treatment with zanubrutinib. Among the events that did recur, none showed a higher grade; indeed, 75.0 % and 40.0 % in the two groups were classified as lower grade. Regarding the efficacy of zanubrutinib, the data showed that ≥ 95 % of the 76 efficacy-evaluable patients across cohorts obtained disease control, with ≥ 65 % achieving partial remission, thereby maintaining or improving response compared to their previous treatment (Table). The safety profile observed during the longer follow-up was consistent with that previously reported for zanubrutinib. As the authors concluded, these longer-term outcomes suggest that patients who are intolerant of other BTK inhibitors can achieve clinical benefit by switching to zanubrutinib. Study enrollment and follow-up are ongoing.
These insights are in keeping with a retrospective observational real-world analysis assessing treatment patterns in patients with relapsed/refractory MCL who started BTK inhibitor therapy between January 2019 and November 2021 in 18 community oncology practices in the United States [6]. Among 402 patients, 44 received zanubrutinib, 161 acalabrutinib and 197 ibrutinib. Although the zanubrutinib group had a relatively shorter mean follow-up than acalabrutinib and ibrutinib (493 vs. 701 and 746 days, respectively) given its later approval date, the zanubrutinib group showed significantly longer median duration of treatment (292 vs. 259 and 149 days, respectively; p < 0.01). Starting from > 60 days, the adherence rates were generally higher for zanubrutinib than for the other two drugs. The discontinuation rate was lowest with zanubrutinib (43.2 % vs. 51.6 % and 45.2 %, respectively). Further analyses on long-term utilization and outcomes are required upon data maturation.
Once-daily vs. twice-daily use
Zanubrutinib is administered at doses of 320 mg OD or 160 mg BID for the treatment of relapsed/refractory MCL, WM, MZL, and CLL/SLL. While both OD and BID doses were assessed in select phase I and II trials, only the BID dose has been used in pivotal clinical studies. Tam et al. reported a comparative summary of clinical data and exposure-response analyses between the two schedules across various B-cell malignancies at EHA 2023 [7]. A total of 216 patients from five studies examining zanubrutinib as monotherapy and in combination with obinutuzumab were identified.
The analysis showed that both 320 mg OD and 160 mg BID are safe and effective, with ORRs ranging from 54.2 % to 100 %. Also, comparable safety profiles were observed with both dosing schedules. Similar rates resulted with respect to grade ≥ 3 hemorrhage, grade ≥ 3 hypertension and grade ≥ 3 atrial fibrillation/flutter, as well as AEs leading to treatment discontinuation. No evident exposure-response relationships were noted between pharmacokinetic parameters (AUC, Cmax, Cmin) and efficacy endpoints or AEs of special interest across indications.
BTK protein degrader BGB-16673
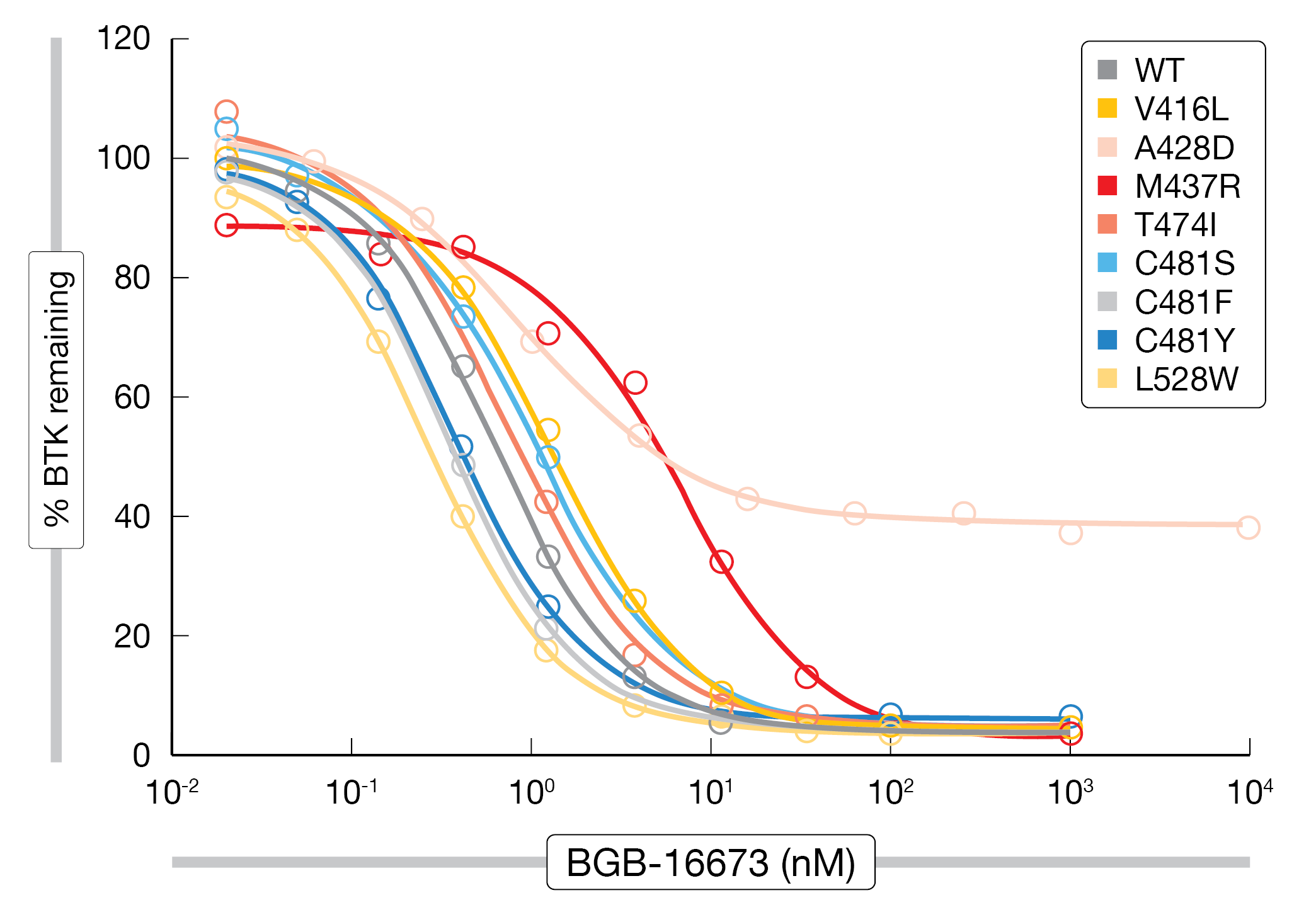
Very little is known about resistance to BTK degraders such as the orally available chimeric degradation-activating compound BGB-16673 that is being investigated in two clinical phase I trials (NCT05006716 and NCT05294731). Feng et al. conducted N-ethyl-N-nitrosourea mutagenesis screening to characterize the profile and tendency of BGB-16673 to cause on-target BTK resistance mutations as compared with the non-covalent BTK inhibitor pirtobrutinib [8]. According to this analysis, BGB-16673 gave rise to fewer resistant clones and showed lower BTK mutation frequency than pirtobrutinib, demonstrating a unique on-target resistance mutation profile. Also, BGB-16673 overcame all BTK resistance mutations from both covalent and non-covalent BTK inhibitor trials except A428D that was resistant to all BTK inhibitors (Figure 2). The new compound degraded BTK in the presence of the clinically relevant resistance mutations V416L, M437R, T474I, C481S, C481F, C481Y, and L528W. As the authors noted, BGB-16673 is a promising novel BTK degrader that could benefit patients who develop BTK inhibitor on-target resistance mutations.
These findings are supported by an analysis conducted based on cell lines and mouse xenograft models [9]. Here, BGB-16673 exhibited high potency on clinically relevant BTK mutants resistant to pirtobrutinib and the covalent BTK inhibitor ibrutinib in cancer cells. The BTK degrader drove complete tumor regression of the lymphoma xenograft models expressing wildtype or BTK mutations that were resistant to the BTK inhibitors. Responses to BGB-16673 lasted longer than those to ibrutinib and pirtobrutinib. Compared to these agents, long-term survival improved with BGB-16673, and metastatic tumor infiltration in spleens was diminished.
Figure 2: Degradation of BTK mutants by the BTK protein degrader BGB-16673
Optimized Bcl-2 inhibition
Bcl-2 inhibition with venetoclax has been established in the treatment of CLL/SLL, although the clinical utility of this drug can be limited by AEs and the development of resistance [10, 11]. BGB-11417, a potent and highly selective Bcl-2 inhibitor, has been designed with the potential to achieve deeper target inhibition and clinical responses [12, 13]. The ongoing phase I BGB-11417-102 study is evaluating BGB-11417 at doses of 80 mg, 160 mg, 320 mg and 640 mg OD in adults with relapsed/refractory B-cell malignancies. Cohort A includes patients with FL, MZL, DLBCL or transformed non-Hodgkin lymphoma, while Cohort B contains patients with CLL/SLL with low tumor burden. Daily and weekly ramp-up schedules were used in Cohorts A and B, respectively, to decrease the risk of tumor lysis syndrome. Li et al. presented the results for 34 and 23 evaluable patients in Cohorts A and B, respectively, at ASCO 2023 [14].
BGB-11417 monotherapy, at all tested doses up to 640 mg, was well tolerated without dose-dependent increases in toxicity. Most TEAEs were grade 1 or 2. In 28.1 %, TEAEs necessitated dose modifications. The most common grade ≥ 3 TEAE was neutropenia (33.3 %), followed by decreases in white blood cell counts (21.1 %) and thrombocytopenia (19.3 %). Dose-limiting toxicities were reported in three patients. No clinical tumor lysis syndrome events occurred.
In Cohort A, six patients (17.6 %) achieved complete or partial responses. Complete remission was observed in two patients both of whom had DLBCL and were treated in the 640 mg cohort. In contrast, responses in Cohort B were observed already at lower dose levels, with 56.5 % showing complete or partial response from 80 mg upwards. Most of these patients remained on study at the time of the analysis (87.0 %), while this proportion was smaller in Cohort A (64.7 %). Among nine MRD-evaluable patients in Cohort B, three had undetectable MRD. Further expansion data are being generated.
REFERENCES
- Nastoupil LJ et al., Ibrutinib plus BR or R-CHOP in previously treated patients with follicular or marginal zone lymphoma: the phase 3 SELENE study. ICML 2023, abstract LBA2
- Stephens DM, Byrd JC, How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood 2019; 133(12): 1298-1307
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940
- Brown JR et al., Zanubrutinib safety/tolerability profile and comparison with ibrutinib profile in B-cell malignancies: post hoc analysis of a large clinical trial safety database. ICML 2023, abstract 339
- Shadman M et al., Updated safety and efficacy results of zanubrutinib in patients with B-cell malignancies who are intolerant of ibrutinib and/or acalabrutinib. EHA 2023, abstract P633
- Shah BD et al., Real-world treatment patterns of Bruton tyrosine kinase inhibitors in patients with mantle cell lymphoma in community oncology practices in the United States. EHA 2023, abstract P1110
- Tam C et al., Efficacy and safety of once daily vs twice daily zanubrutinib for patients with various B-cell malignancies: a comparative summary of clinical data and exposure-response analyses. EHA 2023, abstract PB1926
- Feng X et al., Bruton tyrosine kinase protein degrader BGB-16673 is less apt to cause, and able to overcome variable BTK resistance mutations compared to other BTK inhibitors. EHA 2023, abstract P1239
- Wang H et al., BGB-16673, a BTK degrader, overcomes on-target resistance from BTK inhibitors and presents sustainable long-term tumor regression in lymphoma xenograft models. EHA 2023, abstract P1219
- Davids MS et al., Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018; 24(18): 4371-4379
- Tausch E et al., Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019; 104(9): e434-e437
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Tam CS et al., Preliminary safety and efficacy data from patients with relapsed/refractory B-cell malignancies treated with the novel B-cell lymphoma 2 (BCL2) inhibitor BGB-11417 in monotherapy or in combination with zanubrutinib. ASH 2021, abstract 1419
- Li C et al., A phase 1 study evaluating the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of Bcl-2 inhibitor BGB-11417 in adult patient with mature B-cell malignancies. J Clin Oncol 41, 2023 (suppl 16; abstr 7558)
© 2023 Springer-Verlag GmbH, Impressum
More posts
DLBCL: treatment of elderly patients and relapsed disease
DLBCL: treatment of elderly patients and relapsed disease POLAR BEAR: novel regimen fr
Waldenström macroglobulinemia: findings from ASPEN and BRUIN
Waldenström macroglobulinemia: findings from ASPEN and BRUIN ASPEN: HRQoL for zanubrut
Outcome improvements in relapsed and untreated marginal zone lymphoma
Outcome improvements in relapsed and untreated marginal zone lymphoma Final analysis o
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors
Follicular lymphoma: study results with bispecific antibodies and BTK inhibitors Mosun
Current insights into BTK inhibition and other targeted approaches in CLL
Current insights into BTK inhibition and other targeted approaches in CLL Treatment-na
Preface – ASCO/EHA/ICML 2023
Preface – ASCO/EHA/ICML 2023 © Dirk Gillissen – Arnon P. Kater, MD, PhD, Department o