ALK-targeted adjuvant treatment and perioperative immunotherapy
Approximately 30 % to 40 % of patients with non–small-cell lung cancer (NSCLC) are diagnosed with resectable disease [1, 2]. Depending on the stage, the risk of disease recurrence remains high in spite of treatment [3], which calls for more effective strategies.
ALINA: alectinib in the adjuvant setting
For patients with resectable ALK-positive NSCLC, the guidelines recommend adjuvant platinum-based chemotherapy, while immunotherapy is not recommended [4]. The potent oral ALK tyrosine kinase inhibitor (TKI) alectinib is widely used as first-line treatment of patients with advanced ALK-positive NSCLC. In the open-label, global phase III ALINA trial, adjuvant alectinib was investigated after resection of stage IB (≥ 4 cm) to IIIA ALK-positive NSCLC. Patients were randomized to either alectinib 600 mg BID for two years (n = 130) or platinum-based chemotherapy Q3W for 4 cycles (n = 127). Disease-free survival (DFS) was defined as the primary endpoint. This was tested hierarchically, with DFS assessment in the stage II-IIIA group preceding testing in the intent-to-treat (ITT) population.
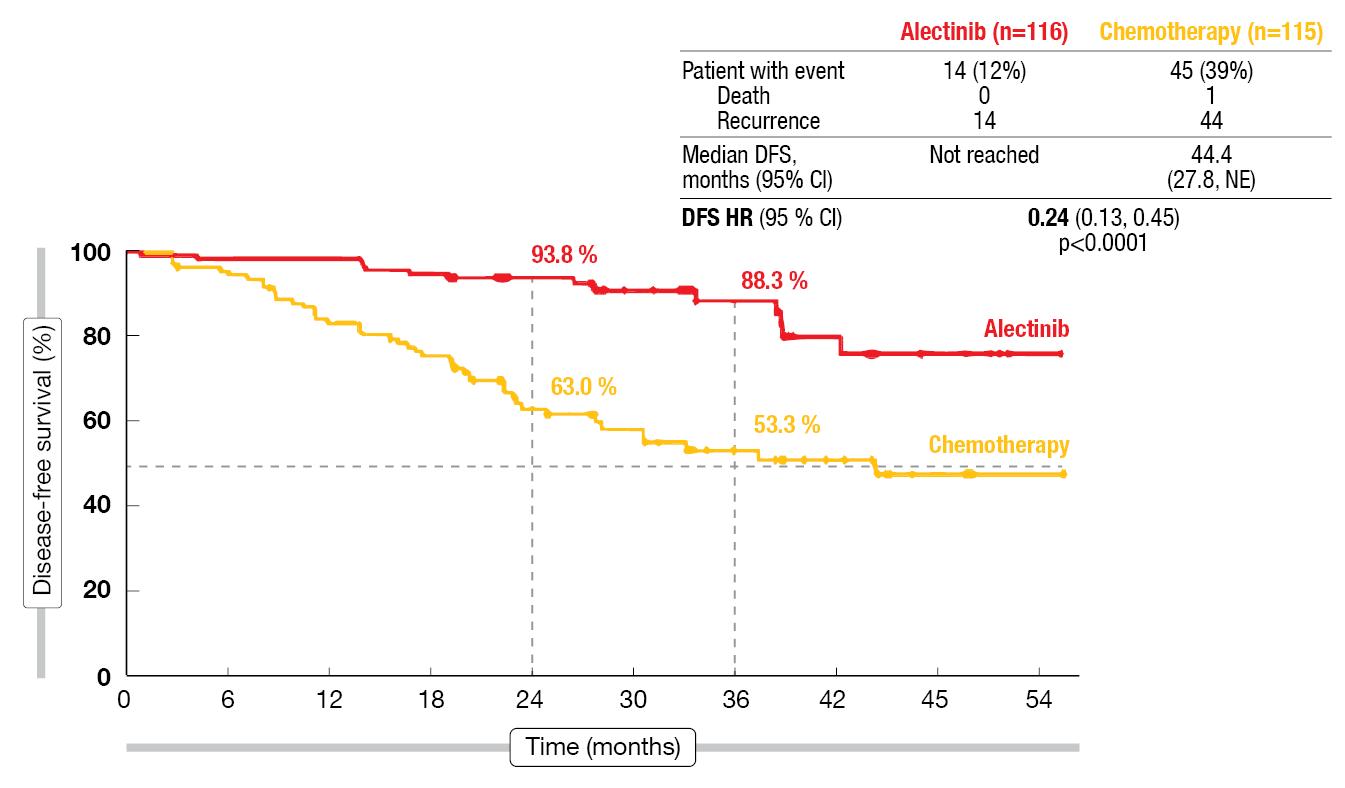
According to the primary results from the pre-specified interim analysis presented at ESMO 2023 by Solomon et al. after a follow-up of 28 months, DFS was significantly improved with alectinib compared to chemotherapy in the stage II-IIIA population [5]. In the experimental arm, median DFS had not been reached, while this was 44.4 months in the control arm (HR, 0.24; p < 0.0001; Figure 1). The 3-year DFS rates were 88.3 % vs. 53.3 %. In the ITT population, the analysis revealed similar results, with median DFS not having been reached and 41.3 months for alectinib and chemotherapy, respectively (HR, 0.24; p < 0.0001). DFS benefits in favor of alectinib were seen across all of the pre-defined subgroups including disease stage and nodal status. CNS disease-free survival, which was an important exploratory endpoint, was longer in the alectinib-treated arm. CNS DFS rates of 95.5 % vs. 79.7 % at 36 months translated into a 78 % risk reduction (HR, 0.22).
In terms of patterns of failure, treatment with adjuvant alectinib resulted in lower proportions of patients with local/regional recurrences (n = 9 vs. 22) and distant recurrences (3 vs. 22). The effect on distant disease was profound, particularly in the brain (4 vs. 14), but also at other sites such as the bone (1 vs. 8). As the safety analysis showed, adjuvant alectinib was tolerable, and the events reported were in keeping with the known safety profile of this TKI. The authors noted that ALINA is the first and only positive phase III trial of an ALK inhibitor in resected, stage IB-IIIA NSCLC, with adjuvant alectinib representing an important new treatment strategy. Other key trials exploring alectinib in stage I-III NSCLC are ongoing, including NAUTIKA1 (NCT04302025), ALNEO (NCT05015010) and HORIZON-01 (NCT05170204).
Figure 1: Improvement of disease-free survival with alectinib vs. chemotherapy in ALINA
<h3>Perioperative checkpoint inhibition: KEYNOTE-671…</h3>
The randomized, double-blind, phase III KEYNOTE-671 trial assessed the perioperative administration of pembrolizumab in addition to chemotherapy in the setting of stage II, IIIA, or IIIB (N2) lung cancer. Prior to surgery, the patients in the experimental arm received neoadjuvant pembrolizumab 200 mg Q3W plus cisplatin/gemcitabine or cisplatin/pemetrexed for up to 4 cycles; this was followed by adjuvant pembrolizumab 200 mg Q3W for up to 13 cycles (n = 397). In the control arm, placebo was administered instead of pembrolizumab (n = 400). According to the first interim analysis, perioperative pembrolizumab plus chemotherapy significantly improved event-free survival (EFS), major pathological response (MPR) and pathological complete response (pCR) compared to neoadjuvant chemotherapy and surgery alone [6].
The second interim analysis of KEYNOTE-671 demonstrated statistically significant, clinically important overall survival (OS) improvement with the pembrolizumab-based regimen after a median follow-up of 36.6 months [7]. Median OS had not been reached in the experimental arm and was 52.4 months in the control arm; at 48 months, 67.1 % vs. 51.5 % of patients were alive (HR, 0.72; p = 0.00517). The OS benefit was generally consistent across the majority of subgroups analyzed. As the authors pointed out, no other perioperative regimen based on immune checkpoint inhibition has previously shown OS improvement in phase III studies of resectable early-stage NSCLC.
In terms of EFS, the advantage that had been observed at the first interim analysis was maintained, with median EFS being almost 2.5 years longer in the immunotherapy-treated patients (47.2 vs. 18.3 months; HR, 0.59). EFS rates at 48 months were 48.4 % vs. 26.2 %. No new safety signals emerged over the prolonged follow-up. Among immune-mediated adverse events (AEs), hypothyroidism represented the most commonly reported event (10.9 % vs. 1.5 %), followed by pneumonitis (6.1 % vs. 1.8 %). However, the incidence of grade 3–5 immune-related events remained low. Based on the results of the KEYNOTE-671 trial, perioperative pembrolizumab has been established as a new standard of care for patients with resectable stage II, IIIA, or IIIB (N2) NSCLC.
<h3>… and CheckMate 77T</h3>
Another study highlighting the merits of perioperative immunotherapy is the global, double-blind phase III CheckMate 77T trial. Patients with resectable, stage IIA (> 4 cm) to IIIB (N2) NSCLC were randomized to either nivolumab 360 mg Q3W plus chemotherapy Q3W for 4 cycles (n = 229) or placebo plus chemotherapy (n = 232). Surgery was performed within six weeks of neoadjuvant treatment. The experimental arm went on to receive nivolumab 480 mg Q4W for one year, while matching placebo was administered in the control arm. A little over half of patients in each arm showed PD-L1 expression ≥ 1 %. EFS by BICR constituted the primary endpoint.
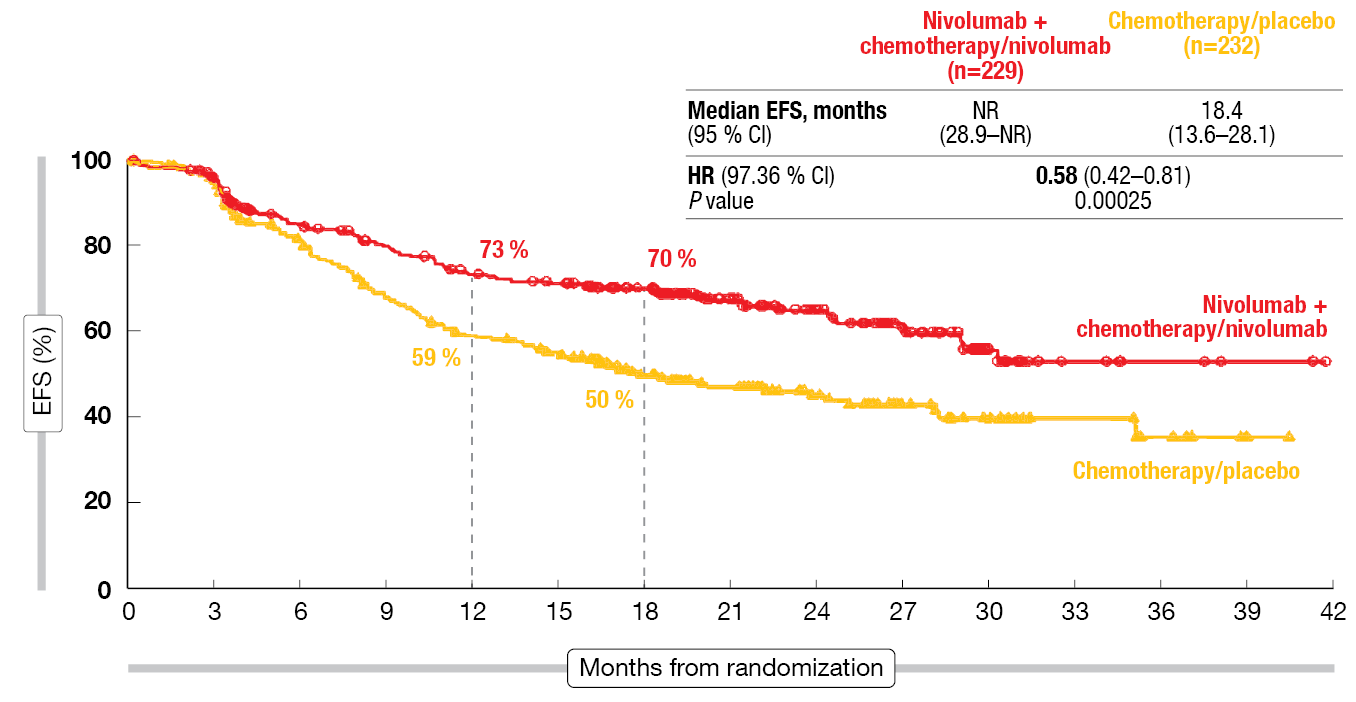
According to the results of the prespecified EFS interim analysis reported by Cascone et al. at ESMO 2023, 78 % and 77 % of the patients in the experimental and control arms, respectively, underwent definitive surgery [8]. In most cases, lobectomy was performed. R0 resection resulted in 90 % in each group. Sixty-two percent vs. 66 % of patients received adjuvant treatment. With respect to the primary endpoint, neoadjuvant nivolumab plus chemotherapy followed by surgery and adjuvant nivolumab improved EFS in a statistically significant and clinically meaningful manner compared to neoadjuvant chemotherapy and surgery alone. Median EFS had not been reached with the nivolumab-based approach and was 18.4 months with chemotherapy and surgery, which translated into a 42 % risk reduction (HR, 0.58; p = 0.00025; <strong>Figure 2</strong>). The experimental regimen performed better across most key subgroups. Patients with stage III disease derived a particularly pronounced EFS benefit (30.2 vs. 13.4 months; HR, 0.51), as did the group with PD-L1 ≥ 1 % (not reached vs. 15.8 months; HR, 0.52).
Furthermore, the addition of immunotherapy gave rise to improvements in the pCR rate (25.3 % vs. 4.7 %; OR, 6.64) and the MPR rate (35.4 % vs. 12.1 %; OR, 4.01). An exploratory analysis indicated EFS improvement with the nivolumab-based regimen compared to chemotherapy alone regardless of pCR status among patients eligible for adjuvant therapy; the HRs for patients with and without pCR were 0.22 and 0.63, respectively. In those unable to receive adjuvant treatment, neoadjuvant nivolumab plus chemotherapy continued to provide EFS benefit over chemotherapy only, with median EFS of 8.8 and 5.2 months, respectively (HR, 0.55). The safety analysis yielded no new signals for perioperative treatment with nivolumab. Feasibility of surgery was similar between the study arms. Taken together, CheckMate 77T supports the perioperative use of nivolumab as a potential new treatment option for patients with resectable NSCLC.
Figure 2: Primary endpoint of CheckMate 77T: event-free survival benefit with perioperative nivolumab
CheckMate 816: 3-year results by PD-L1 expression
In the phase III CheckMate 816 study, the addition of nivolumab to neoadjuvant chemotherapy has shown statistically significant and clinically meaningful improvements in EFS and pCR compared to chemotherapy alone [9]. Provencio Pulla et al. presented prespecified exploratory subgroup analyses that explored the outcomes in patients with PD-L1 ≥ 1 % or < 1 % in CheckMate 816 [10].
The findings demonstrated that the combination provides clinical benefit compared to chemotherapy alone irrespective of tumor PD-L1 expression, although the magnitude of benefit was comparatively greater in the PD-L1–positive group. Patients with PD-L1 ≥ 1 % showed pCR rates of 32.6 % vs. 2.2 % for nivolumab plus chemotherapy vs. chemotherapy alone, whereas the pCR rates were 16.7 % vs. 2.6 % for those with PD-L1 < 1 %. Median EFS had not been reached and was 26.7 months in the PD-L1–positive group (HR, 0.46); in the absence of PD-L1 positivity, this was 26.4 vs. 20.8 months (HR, 0.87). At 36 months, 85 % vs. 66 % of patients with PD-L1 ≥ 1 % were alive (HR, 0.37), while these proportions were 71 % vs. 60 % in those with PD-L1 < 1 % (HR, 0.81). In both treatment arms, patients achieving pCR experienced improved EFS and OS compared to those without pCR.
Neoadjuvant nivolumab plus chemotherapy exhibited a manageable safety profile and did not impact the feasibility of surgery compared to chemotherapy alone, irrespective of tumor PD-L1 expression. The authors concluded that these findings reinforce the role of nivolumab plus chemotherapy as a standard neoadjuvant approach for eligible patients with resectable NSCLC and tumor PD-L1 expression ≥ 1 % or < 1 %.
Responses to neoadjuvant tislelizumab
The perioperative use of the PD-1 inhibitor tislelizumab is being assessed in the randomized, double-blind phase III RATIONALE-315 study that is conducted in patients with resectable stage II-IIIA NSCLC. Neoadjuvant tislelizumab 200 mg Q3W plus platinum-doublet chemotherapy is administered for 3–4 cycles prior to surgery (n = 226). In the adjuvant phase, the treatment consists of tislelizumab 400 mg Q6W for up to 8 cycles. The group randomized to the control arm receives neoadjuvant chemotherapy alone, and placebo is used instead of the PD-1 inhibitor before and after surgery (n = 227). Primary endpoints include the MPR rate by blinded independent pathological review and EFS by BICR. pCR constitutes the key secondary endpoint. At ESMO 2023, Yue et al. reported the MPR and pCR findings after a median follow-up of 16.8 months [11].
Neoadjuvant tislelizumab plus chemotherapy, as compared to chemotherapy only, induced statistically significant and clinically meaningful improvement of both MPR (56.2 % vs. 15.0 %; OR, 7.5; p < 0.0001) and pCR (40.7 % vs. 5.7 %; OR, 11.5; p < 0.0001). The safety profile of the combination was consistent with the known risks of each component, and the treatment was well tolerated. Median duration of treatment was similar across the arms, as was the number of cycles received. The RATIONALE-315 study is ongoing, and further data will be shared at future meetings.
REFERENCES
- Cagle PT et al., Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med 2013; 137(9): 1191-1198
- Le Chevalier T, Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol 2010; 21 Suppl 7: vii196-98
- Pignon JP et al., Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-3559
- NCCN Clinical Practice Guidelines in Oncology, NSCLC v.3 2023
- Solomon BJ et al., ALINA: efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ NSCLC. ESMO 2023, abstract LBA2
- Wakelee H et al., Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 2023; 389(6): 491-503
- Spicer JD et al., OS in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage NSCLC. ESMO 2023, abstract LBA56
- Cascone T et al., CheckMate 77T: Phase 3 study comparing neoadjuvant nivolumab plus chemotherapy with neoadjuvant placebo plus chemotherapy followed by surgery and adjuvant nivolumab or placebo for previously untreated, resectable stage II-IIIB NSCLC. ESMO 2023, abstract LBA1
- Forde PM et al., Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386(21): 1973-1985
- Provencio Pulla M et al., Neoadjuvant nivolumab plus chemotherapy in the phase 3 CheckMate 816 study: 3-year results by tumor PD-L1 expression. ESMO 2023, abstract LBA57
- Yue D et al., Pathological response to neoadjuvant tislelizumab plus platinum-doublet chemotherapy in resectable stage II-IIIA NSCLC patients in the phase 3 RATIONALE-315 trial. ESMO 2023, abstract LBA58
© 2023 Springer-Verlag GmbH, Impressum