Immunotherapy combinations in advanced-stage disease
As is known, immune checkpoint inhibition plays only a limited role after failure of EGFR- or ALK-targeted treatment in patients with advanced NSCLC; this applies to both monotherapy and combinations with chemotherapy as demonstrated by the CheckMate 722 and KEYNOTE-789 trials [1, 2]. It was hypothesized that inhibition of VEGF might enhance the activity of chemoimmunotherapy by increasing lymphocyte trafficking to the tumor microenvironment and reversing VEGF-mediated immunosuppression [3, 4]. Indeed, a subgroup analysis of the phase III IMpower150 study has shown improved efficacy of the PD-L1 inhibitor atezolizumab in combination with anti-angiogenic therapy, particularly in EGFR-mutated NSCLC [5].
ATTLAS: ABCP vs. PC
Therefore, the multicenter, open-label, randomized phase III ATTLAS study was initiated to evaluate atezolizumab plus the anti-VEGF antibody bevacizumab in addition to chemotherapy with carboplatin and paclitaxel (ABCP) in the setting of stage IV, non-squamous NSCLC harboring activating EGFR or ALK alterations after progression on ≥ 1 EGFR or ALK tyrosine kinase inhibitors (TKIs). In the absence of EGFR T790M mutations, EGFR TKIs of the first or second generation had been used, while third-generation TKI pretreatment was required in patients with T790M mutations. Induction treatment in the experimental arm entailed the administration of ABCP Q3W for 4 to 6 cycles (n = 154). This was followed by the maintenance phase consisting of atezolizumab plus bevacizumab Q3W until disease progression or loss of clinical benefit. Patients in the control arm (n = 74) received chemotherapy alone, i.e., pemetrexed plus carboplatin or cisplatin (PC) Q3W for 4 to 6 cycles followed by pemetrexed maintenance Q3W until disease progression or loss of clinical benefit. Progression-free survival (PFS) was the primary endpoint. Brain metastases were present in more than 40 % of patients in each study arm.
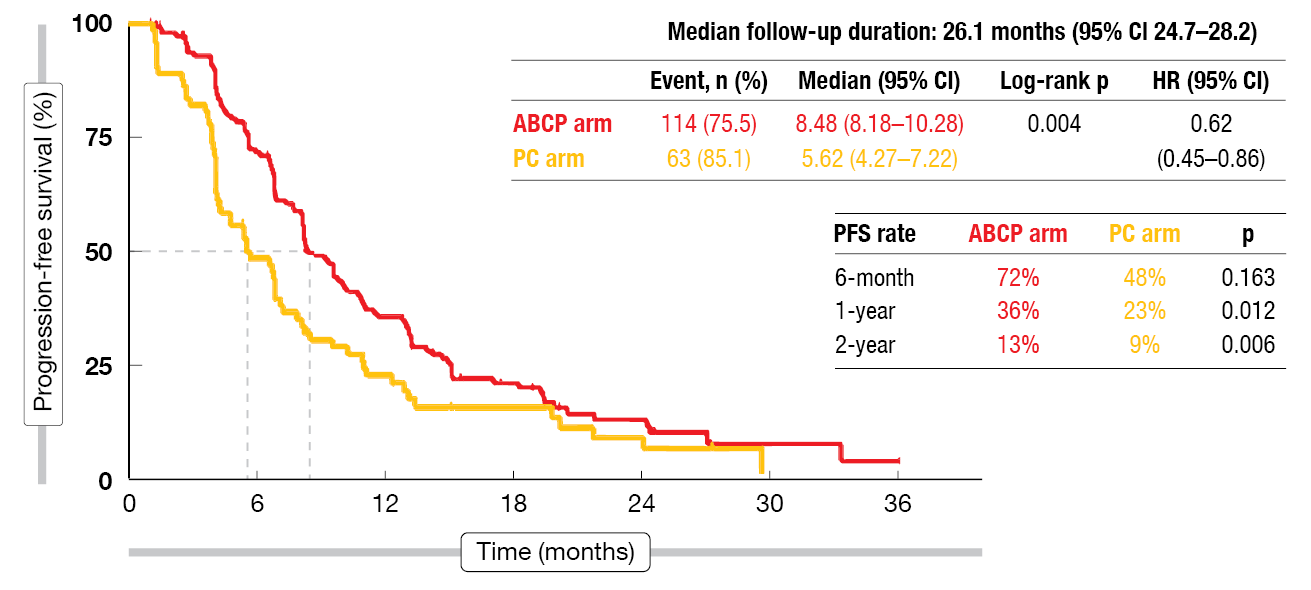
Results from the first analysis of ATTLAS were reported at ESMO 2023 by Ahn et al. [6]. The ABCP regimen, as compared to PC, gave rise to a statistically significant and clinically meaningful PFS improvement (8.48 vs. 5.62 months; HR, 0.62; p = 0.004; Figure 1). At 12 months, 36 % vs. 23 % of patients were progression-free (p = 0.012). Particularly large risk reductions resulted in the subgroups with brain metastases (HR, 0.32), L858R mutation (HR, 0.52) and those without acquired T790M mutations (HR, 0.44). According to an exploratory analysis, the PFS benefit of ABCP was greatest in the patients showing the highest PD-L1 expression (TPS ≥ 50 %) as well as in those with inflamed scores ≥ 20 % vs. < 20 %.
No difference was observed regarding overall survival (OS; 20.63 vs. 20.27 months; HR, 1.01; p = 0.975). Objective responses resulted in 69.5 % vs. 41.9 % and disease control in 96.7 % vs. 87.8 %. The median best reductions in target lesion size were -43.8 % vs. -26.0 % with ABCP vs. PC. Treatment-related adverse events (AEs) occurred more commonly with ABCP than PC, as did dose interruptions or modifications and permanent discontinuation. However, AEs proved manageable, and no new safety signals emerged during the study. Taken together, the data obtained in the ATTLAS trial showed that the addition of atezolizumab and bevacizumab to chemotherapy should be considered a feasible option in patients with NSCLC harboring activating EGFR or ALK alterations after progression on targeted treatment.
Figure 1: ATTLAS study: improvement of progression-free survival with atezolizumab, bevacizumab and paclitaxel/carboplatin (ABCP) compared to pemetrexed plus platinum (PC)
OS findings with dostarlimab plus chemotherapy
The anti-PD-1 antibody dostarlimab that binds to PD-1 at distinct bindings sites with different binding orientations from other PD-1 inhibitors has been shown to significantly improve outcomes in rectal and endometrial cancer [7-9]. PERLA was the first global, randomized, double-blind head-to-head study comparing dostarlimab with pembrolizumab in the setting of NSCLC. In this phase II trial, patients with untreated metastatic non-squamous NSCLC devoid of known targetable oncogenic driver aberrations received either dostarlimab 500 mg Q3W plus chemotherapy (n = 121) or pembrolizumab 200 mg Q3W plus chemotherapy (n = 122). The overall response rate (ORR) by blinded independent review was defined as the primary endpoint based on the scientists’ hypothesis that the ORRs might be similar across the two regimens. According to the primary analysis, PERLA met its primary endpoint, demonstrating favorable numerical trends in ORR and PFS for the dostarlimab-based regimen [10]. Peters et al. presented the pre-planned updated OS results at ESMO 2023 [11].
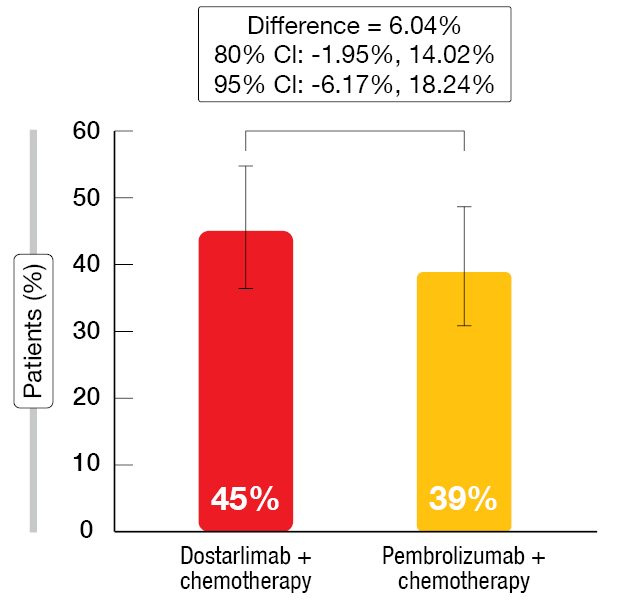
Dostarlimab plus chemotherapy continued to exhibit strong clinical efficacy. Consistent with the primary analysis, the ORR remained numerically higher in the experimental arm (45 % vs. 39 % Figure 2). Median duration of exposure was longer in the dostarlimab-treated group (9 vs. 6 months), with a median of 13 vs. 7.5 cycles administered. After a median follow-up of 21 months, a trend favored the dostarlimab-based strategy in terms of OS (19.4 vs. 15.9 months; HR, 0.75). This numerical superiority held true in all patients with positive PD-L1 assessment (PD-L1 TPS ≥ 1 %). Also, median PFS was longer for dostarlimab plus chemotherapy than pembrolizumab plus chemotherapy in the group without PD-L1 expression (TPS < 1 %), although the Kaplan Meier curves crossed twice.
Similar proportions of patients across the two arms experienced AEs and grade ≥ 3 AEs. In the experimental arm, fewer patients developed AEs leading to treatment discontinuation, serious AEs and immune-related AEs. In their summary, the authors pointed out that these updated results are consistent with the study hypothesis that dostarlimab and pembrolizumab have similar efficacy. These findings support further investigation of dostarlimab as a backbone in combination with standard-of-care regimens.
Figure 2: Confirmed overall response rates with dostarlimab/chemotherapy vs. pembrolizumab/chemotherapy
Negative results for sitravatinib/nivolumab
No advantage was found for the combination of the multi-kinase inhibitor sitravatinib with nivolumab that was tested against docetaxel in the phase III SAPPHIRE study [12]. Patients with unresectable, locally advanced or metastatic non-squamous NSCLC that had no actionable genomic alterations participated in the trial after one or two prior regimens. The most recent regimen included immune checkpoint inhibition with or after platinum-based chemotherapy. Based on the observation that checkpoint inhibitor resistance is driven by an immunosuppressive tumor microenvironment (TME), it was hypothesized that sitravatinib might shift the TME towards a less immunosuppressive state and thus improve outcomes when administered in combination with nivolumab after failure of checkpoint inhibition.
OS, which was the primary objective, did not differ significantly for sitravatinib/nivolumab vs. docetaxel (12.2 vs. 10.6 months; HR, 0.86; p = 0.144), which also applied to PFS (4.4 vs. 5.4 months; HR, 1.08; p = 0.452), ORR (16 % vs. 17 %; p = 0.597) and duration of response (7.4 vs. 7.1 months; p = 0.924). None of the subgroups appeared to benefit from the combination in a notable manner; indeed, never smokers and patients who had previously received sequential checkpoint inhibition and platinum-based chemotherapy fared better with docetaxel. The safety profiles for sitravatinib/nivolumab and docetaxel were consistent with the known profiles. Immune-related AEs of any grade occurred in 46 % of combination-treated patients, the most frequent being hypothyroidism (14 %) and diarrhea (12 %). The authors concluded that further studies are needed to identify treatment options for patients with NSCLC who have developed resistance against immune checkpoint inhibition.
REFERENCES
- Mok TSK et al., Nivolumab + chemotherapy vs. chemotherapy in patients with EGFR-mutated metastatic non-small cell lung cancer with disease progression after EGFR tyrosine kinase inhibitors in CheckMate 722. ESMO Asia 2022, abstract LBA8
- Yang JC-H et al., Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: phase 3 KEYNOTE-789 study. J Clin Oncol 2023; 41(suppl 17): LBA9000
- Chen D, Mellman I, Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39(1): 1-10
- Hegde PS et al., Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018; 52(Pt 2): 117-124
- Reck M et al., Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2023; 7(5): 387-401
- Ahn MJ et al., A phase 3, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR and ALK mutated in non-small cell lung cancer. ESMO 2023, LBA67
- Park UB et al., Molecular basis of PD-1 blockade by dostarlimab, the FDA-approved antibody for cancer immunotherapy. Biochem Biophys Res Commun 2022; 599: 31-37
- Cercek A et al., PD-1 Blockade in mismatch repair-deficient, locally advanced rectal cancer.
N Engl J Med 2022; 386(25): 2363-2376 - Mirza MR et al., Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 2023; 388(23): 2145-58
- Peters S et al., Randomized double-blind phase II trial (PERLA) of dostarlimab + chemotherapy vs pembrolizumab + chemotherapy in metastatic non-squamous NSCLC: primary results. Ann Oncol 2022; 16(suppl_1): 100102–100102
- Peters S et al., Overall survival from a phase II randomized double-blind trial (PERLA) of dostarlimab + chemotherapy vs pembrolizumab + chemotherapy in metastatic non-squamous NSCLC. ESMO 2023, LBA64
- Borghaei H et al., SAPPHIRE: phase 3 study of sitravatinib plus nivolumab versus docetaxel in patients with previously treated advanced non-squamous non-small cell lung cancer. ESMO 2023, abstract LBA63
© 2023 Springer-Verlag GmbH, Impressum