Innovative agents directed against RET, Trop-2, KRASG12C and HER2
Superiority of selpercatinib in RET-positive disease
The highly selective and potent RET kinase inhibitor selpercatinib has been implemented in the treatment of lung cancer harboring RET gene fusions. At the same time, the combination of platinum, pemetrexed and pembrolizumab is an established first-line standard of care for patients without EGFR or ALK alterations. The aim of the randomized, open-label, phase III LIBRETTO-431 study was to define the optimal first-line regimen for patients with RET-fusion–positive NSCLC. Selpercatinib 160 mg BID (n = 129) was compared with carboplatin or cisplatin plus pemetrexed with or without pembrolizumab (n = 83) in the setting of untreated stage IIIB-IIIC or IV non-squamous, RET-positive NSCLC. Progression-free survival (PFS) by blinded independent central review (BICR) in the ITT-pembrolizumab population receiving chemotherapy plus pembrolizumab as well as the overall ITT population constituted the gated primary endpoints. Crossover from chemotherapy to selpercatinib was possible upon BICR-confirmed disease progression. A total of 103 centers in 23 countries participated in LIBRETTO-431.
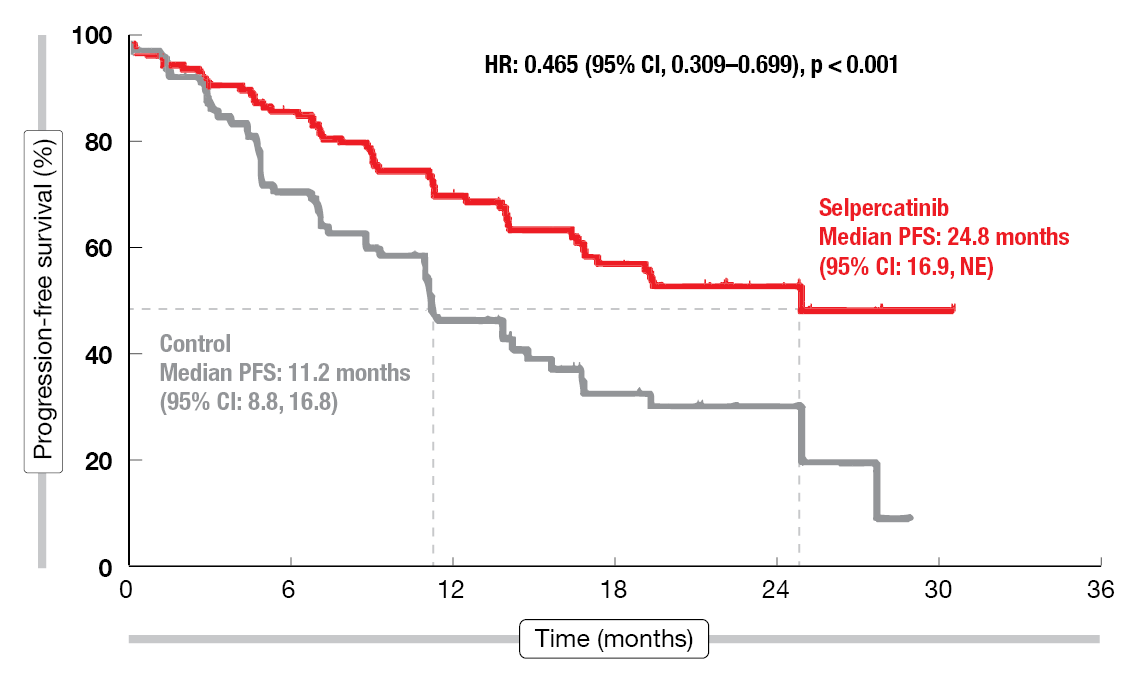
According to the results of the protocol-specified interim analysis reported at ESMO 2023 by Loong et al., selpercatinib outperformed the control regimens by a considerable margin [1]. PFS was longer in a statistically significant and clinically meaningful manner in both the ITT-pembrolizumab population (24.8 vs. 11.2 months; HR, 0.465; p < 0.001; Figure 1) and the ITT population (24.8 vs. 11.2 months; HR, 0.482; p < 0.001). Consistent PFS benefits were observed across all preplanned subgroups; this involved superior outcomes in the selpercatinib arm independent of PD-L1 expression status. Furthermore, selpercatinib elicited a higher overall response rate than the control regimens (83.7 % vs. 65.1 %), and responses were more durable (24.2 vs. 11.5 months). Overall survival (OS) results were immature and confounded by the crossover (n = 42).
Figure 1: Progression-free survival with selpercatinib vs. platinum plus pemetrexed ± pembrolizumab
Improved CNS disease control
Asymptomatic brain metastases were present at baseline in approximately 20 % of patients in each arm. In this population, selpercatinib demonstrated improvements in terms of intracranial responses (82.4 % vs. 58.3 %) and intracranial PFS (16.1 vs. 10.4 months). Intracranial complete remissions resulted in 35.3 % vs. 16.7 %. Time to CNS progression was delayed with selpercatinib therapy. In patients both with and without baseline CNS metastases, the cumulative incidence of CNS progression was lower at 12 months (5.5 % vs. 20.3 %; cause-specific HR, 0.28). The 12-month cumulative incidence rates in the group with CNS lesions at baseline were 25.7 % vs. 33.3 % (cause-specific HR, 0.61). For the cohort without CNS metastases, this was 1.1 % vs. 14.7 % (cause-specific HR, 0.17).
The treatment-emergent adverse events (TEAEs) observed in the experimental arm were generally consistent with those previously reported and were mostly managed with dose modification. Transaminase elevations occurred as the most frequent AEs, followed by hypertension, diarrhea, edema, and dry mouth. Median time on selpercatinib treatment was approximately 70 % longer than time on control treatment (16.7 vs. 9.8 months). Assessments of pulmonary symptoms and physical function using the NSCLC Symptom Assessment Questionnaire and the EORTC QLQ-C30 showed that selpercatinib, as compared to chemo(immuno)therapy, delayed the time to deterioration of pulmonary symptoms (HR, 0.34) and overall physical function (HR, 0.60). In their summary, the authors noted that selpercatinib should be considered a first-line standard of care in RET-fusion–positive advanced NSCLC. Also, these results reinforce the importance of genomic testing to identify RET fusions at the time of diagnosis to inform initial therapy.
Dato-DXd in patients with genomic alterations
Standard-of-care second-line chemotherapy for metastatic NSCLC shows only modest clinical benefit and substantial toxicity. Therefore, innovative approaches such as the Trop-2-directed antibody drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd) are being explored in pretreated patients with and without oncogenic drivers. Dato-DXd 6 mg/kg Q3W was tested in the single-arm, phase II TROPION-Lung05 study in 137 patients with stage IIIB, IIIC, or IV NSCLC and at least one actionable genomic alteration (i.e., EGFR, ALK, ROS1, NTRK, BRAF, MET exon 14 skipping, RET). They had received ≥ 1 line of targeted treatment and 1 or 2 prior cytotoxic agent-containing therapies including platinum-based regimens in the metastatic setting; the median number of prior lines in advanced disease was 3. Radiographic disease progression had occurred after targeted therapy. EGFR mutations were present in 57 %, followed by ALK rearrangement (25 %). Half of the total population showed a history of brain metastasis. The objective response rate (ORR) by BICR constituted the primary endpoint.
Dato-DXd demonstrated encouraging antitumor activity in this heavily pretreated NSCLC population [2]. The confirmed ORR was 35.8 % in all treated patients; in the groups with EGFR mutations and ALK rearrangement, this was 43.6 % and 23.5 %, respectively. Median duration of response was 7.0 months across the groups. In the total population, complete and partial remissions resulted in 3 % and 33 %, respectively, and disease control was obtained in 78.8 %. Median PFS was 5.4 months overall; for the groups with EGFR mutations and ALK rearrangement, this was 5.8 and 4.3 months, respectively. A subset analysis of 68 patients with sensitizing mutations or T790M mutations showed that individuals previously treated with osimertinib achieved an ORR of 49.1 %.
Nausea, stomatitis and alopecia were reported as the most common TEAEs. The safety profile was characterized by low incidences of hematologic AEs and drug-related grade ≥ 3 toxicities. TEAEs necessitated dose reductions and dose withdrawal in 22 % and 10 %, respectively. In 2 %, TEAEs associated with death were reported. AEs of special interest included oral mucositis/stomatitis (all grades, 66 %), ocular surface toxicity (26 %), infusion-related reactions (16 %), and interstitial lung disease (ILD; 4 %). All of these were mostly grade 1 and 2.
TROPION-Lung01: Dato-DXd vs. chemotherapy
The randomized, phase III, open-label, global TROPION-Lung01 study is currently comparing Dato-DXd 6 mg/kg Q3W (n = 299) with docetaxel 75 mg/m2 Q3W (n = 305) in stage IIIB, IIIC or IV NSCLC with or without actionable genomic alterations. Pretreatment consisted of 1 or 2 lines including platinum chemotherapy and immunotherapy in the group without driver aberrations, and 1 or 2 approved targeted agents plus chemotherapy and ≤ 1 anti-PD-(L)1 antibody in the group harboring driver aberrations. Actionable genomic alterations were found in 17 % in both arms, with EGFR mutation rates of 13 % and 15 % in the Dato-DXd and docetaxel groups, respectively. Squamous histology was present in 22 % and 23 %, respectively. Progression-free survival (PFS) by BICR and OS were defined as the dual primary endpoints.
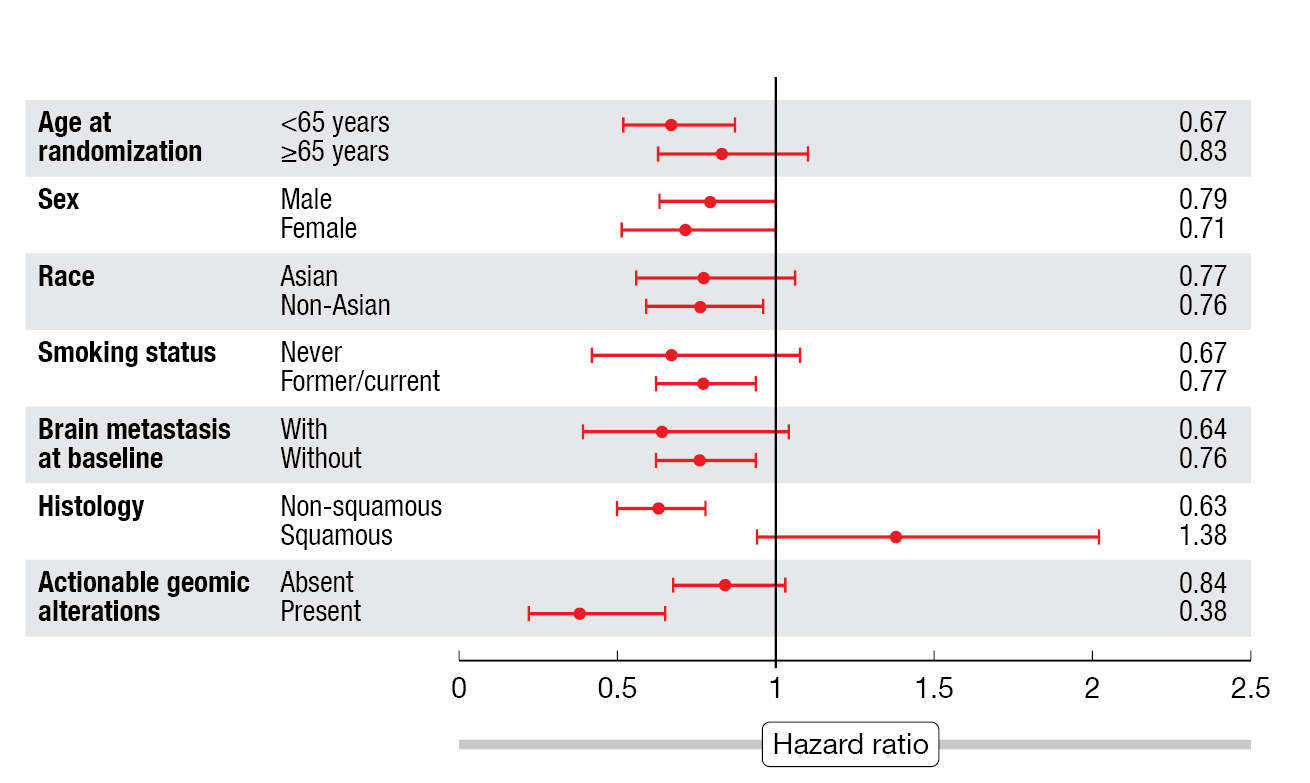
According to the results presented at ESMO 2023, Dato-DXd, as compared to docetaxel, induced a 25 % reduction in the risk of progression or death, with median PFS of 4.4 vs. 3.7 months (HR, 0.75; p = 0.004) [3]. ORRs were 26.4 % vs. 12.8 %, and responses lasted for a median of 7.1 vs. 5.6 months. The PFS benefit was mainly driven by the group with non-squamous histology that derived a 37 % risk reduction (5.6 vs. 3.7 months; HR, 0.63; Figure 2). Likewise, the patients with non-squamous histology showed a 23 % reduction in mortality risk (HR, 0.77), while no difference was noted for OS in the overall group (12.4 vs. 11.0 months; HR, 0.90).
No new safety signals emerged in the TROPION-Lung01 study. Stomatitis and nausea were the most frequent treatment-related AEs (TRAEs) in the experimental arm and were predominantly grade 1 or 2. Fewer grade ≥ 3 TRAEs occurred with Dato-DXd than with chemotherapy (25 % vs. 41 %). Among AEs of special interest, the analysis yielded adjudicated drug-related ILD rates of 8 % vs. 4 %, with 3 % vs. 1 % classified as grade ≥ 3. Grade 5 ILD events were reported in 2 % (n = 7) vs. 0.3 % (n = 1). This highlights the need for careful monitoring and adherence to ILD management guidelines, as the authors stressed. Infusion-related reactions were noted in 8 % of patients in each arm; with the exception of one grade 3 event in the experimental arm, all of these were grade ≤ 2. Overall, Dato-DXd is the first ADC to demonstrate a statistically significant improvement in PFS over docetaxel in patients with pretreated, locally advanced or metastatic NSCLC. Dato-DXd constitutes a potential new therapy in the setting of previously treated non-squamous disease.
Figure 2: TROPION-Lung01 trial: progression-free survival in key subgroups
Adagrasib in addition to pembrolizumab
The KRASG12C inhibitor adagrasib has been designed to show favorable properties including long half-life and dose-dependent pharmacokinetics, CNS penetration, and non-covalent binding affinity as well as minimized cysteine reactivity [4, 5]. These features are assumed to limit off-target effects in the liver and other organs. Indeed, in contrast to sotorasib, adagrasib can be administered concurrently or sequentially with pembrolizumab without severe hepatotoxicity impeding its use [6, 7].
Adagrasib 400 mg BID plus concurrent pembrolizumab 200 mg Q3W was investigated as first-line treatment in the phase II cohorts 1a and 2 of the single-arm KRYSTAL-7 study that enrolled patients with advanced, unresectable or metastatic NSCLC harboring KRASG12C mutation. Stable brain metastases were allowed. ORR was defined as the primary endpoint. At ESMO 2023, Garassino et al. reported safety in all treated patients (n = 148) and efficacy in patients with PD-L1 TPS ≥ 50 % (n = 51) after median follow-up of 8.7 and 10.1 months for all patients and those with PD-L1 TPS ≥ 50 %, respectively [8].
The combination of adagrasib and pembrolizumab showed a manageable safety profile that was consistent with either agent as monotherapy. Most commonly, nausea (any grade, 51 %) and diarrhea (44 %) occurred, as well as ALT and AST increases (38 % and 32 %, respectively). Treatment-related grade ≥ 3 elevations of ALT and AST were observed in 24 patients (16 %). Ten of these received concomitant steroids, and most were able to resume the combination treatment. Despite transaminase elevations, treatment-related hepatic events were limited to < 10 % of patients. No patient discontinued either adagrasib or pembrolizumab due to transaminase increases or hepatic TRAEs. Two grade 5 TRAEs that included pneumonia and pneumonitis were reported. Immune-related TRAEs of any grade emerged in 18 %, with grade ≥ 3 events noted in 5 %. Adagrasib dose reductions and temporary dose interruption due to TRAEs were performed in 46 % and 59 % of patients, respectively. Permanent discontinuation of adagrasib or pembrolizumab resulted in 6 % and 11 %, respectively, and in 4 %, both drugs were discontinued due to TRAEs.
Encouraging preliminary efficacy was seen in the group with PD-L1 TPS ≥ 50 % that showed an ORR of 63 % and disease control in 84 %. This was higher than expected with pembrolizumab monotherapy (39-45 %) [9, 10]. In patients who experienced any-grade hepatotoxicity, the ORR was 70 %. Moreover, the data suggested promising early signs of durability. Median duration of response had not been reached at the time of the analysis, which was also true for median PFS. The authors noted that these findings support the initiation of a phase III trial evaluating concurrent adagrasib plus pembrolizumab compared to pembrolizumab in treatment-naïve KRASG12C-mutated NSCLC with PD-L1 TPS ≥ 50 %.
Outcomes with T-DXd by presence of brain lesions
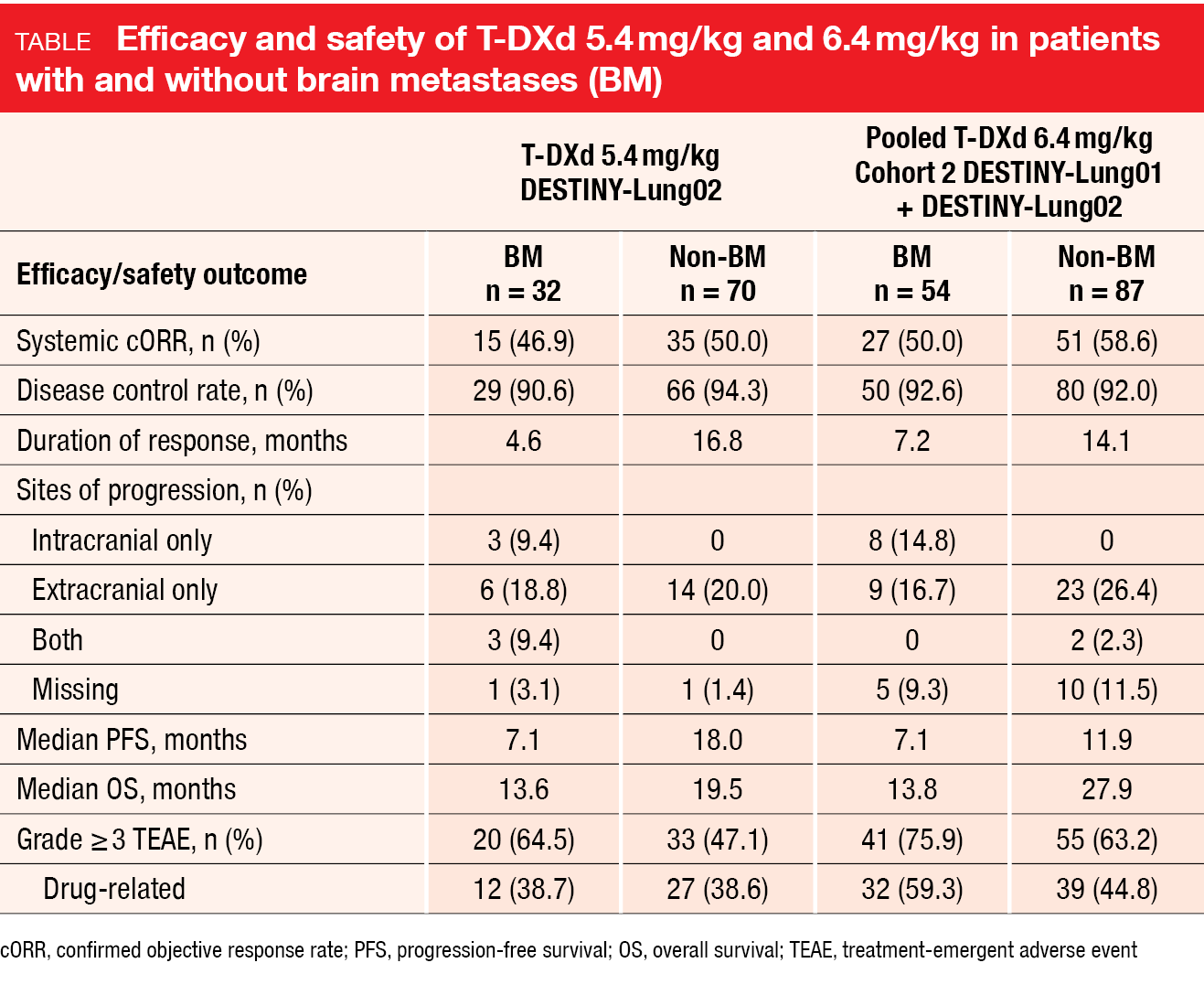
In the setting of advanced or metastatic HER2-mutant NSCLC, the ADC trastuzumab deruxtecan (T-DXd) has been evaluated in the DESTINY-Lung02 trial and in Cohort 2 of the DESTINY-Lung01 study. Li et al. presented results for patients with and without brain metastases (BM) who received T-DXd 5.4 mg/kg Q3W in DESTINY-Lung02 (32 and 70 with and without BM, respectively) or T-DXd 6.4 mg/kg Q3W in Cohort 2 of DESTINY-Lung01 and in DESTINY-
Lung02 (54 and 87 with and without BM, respectively) [11]. Regarding systemic responses, the analyses yielded similar results independent of the presence of BM (Table). Confirmed ORRs ranged from 46.9 % to 58.6 % across dose levels and cohorts with and without BM, and disease control rates exceeded 90 % in all groups. Median PFS was shorter in the BM cohorts compared to the non-BM groups; this also applied to median OS.
In the population with BM at baseline, T-DXd demonstrated intracranial efficacy. Reductions in BM size from baseline as best overall response were observed in 86 % and 78 % for T-DXd 5.4 mg/kg and 6.4 mg/kg, respectively. Intracranial ORRs were 50 % and 30 %, respectively, and included complete responses in three patients treated with the lower dose (21.4 %). In 92.9 % and 73.3 %, respectively, intracranial disease control was achieved. Median duration of intracranial response was 9.5 and 4.4 months, respectively. Notably, patients with treated and untreated BMs experienced comparable intracranial responses.
The safety outcomes were similar regardless of the presence of BM, although patients with BM had higher rates of grade ≥ 3 and serious TEAEs than those without. The authors noted that limitations of this post-hoc analysis include the small number of patients and the lack of a comparator arm.
Encouraging results in Beamion LUNG-1
Zongertinib, an oral tyrosine kinase inhibitor that covalently and selectively binds to the tyrosine kinase domain of HER2 while sparing wild-type EGFR, is being tested in the multicohort phase Ia/Ib Beamion LUNG-1 study in the setting of NSCLC and other cancers harboring HER2 aberrations. Phase Ia includes patients with advanced solid tumors who have exhausted existing standard options or are not suitable for them. HER2 aberrations comprise overexpression, amplification, somatic mutation, and gene rearrangement involving HER2 or NRG1. In phase Ib, patients with HER2-mutated NSCLC are enrolled into five cohorts.
According to the latest data presented at ESMO 2023, the planned futility analysis was passed and the Beamion LUNG-1 study is continuing, with recruitment into all cohorts ongoing [12]. The maximum tolerated dose of zongertinib was not reached in phase Ia. The doses taken into dose optimization were 240 mg and 120 mg OD. Initial efficacy results were encouraging; in phase Ia, the ORR and the disease control rate in NSCLC patients were 50.0 % and 97.1 %, respectively, and in phase Ib, this was 73.9 % and 91.3 %, respectively. Zongertinib was well tolerated, with low rates of EGFR-
mediated AEs and no cases of drug discontinuation due to AEs in phase Ib. All responders were ongoing at the time of the analysis.
REFERENCES
- Loong HH et al., Randomized phase 3 study of first-line selpercatinib versus chemotherapy and pembrolizumab in RET fusion-positive NSCLC. ESMO 2023, abstract LBA4
- Paz-Ares L et al., TROPION-Lung05: Datopotamab deruxtecan in previously treated non-small cell lung cancer with actionable genomic alterations. ESMO 2023, abstract 1314MO
- Lisberg A et al., Datopotamab deruxtecan vs docetaxel in previously treated advanced/metastatic non-small cell lung cancer: Results of the randomized phase III study TROPION-Lung01. ESMO 2023, abstract LBA12
- Hallin J et al., The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 2020; 10(1): 54-71
- Ou S-HI et al., First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid
tumors (KRYSTAL-1). J Clin Oncol 2022; 23(40): 2530-2538 - Gadgeel SM et al., KRYSTAL-1: Two-year follow-up of adagrasib (MRTX849) monotherapy in patients with advanced/metastatic KRASG12C-mutated NSCLC. WCLC 2023, abstract MA06.04
- Jänne PA et al., Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 2022; 387(2): 120-131
- Garassino MC et al., KRYSTAL-7: Efficacy and safety of adagrasib with pembrolizumab in patients with treatment-naïve, advanced non-small cell lung cancer harboring a KRASG12C mutation. ESMO 2023, abstract LBA65
- Mok TSK et al., Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393(10183): 1819-1830
- Reck M et al., Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375(19): 1823-1833
- Li BT et al., Trastuzumab deruxtecan in patients with HER2(ERBB2)-mutant metastatic non-small cell lung cancer with and without brain metastases: exploratory pooled analyses from DESTINY-Lung01 and DESTINY-Lung02. ESMO 2023, abstract 1321MO
- Ruiter G et al., Beamion LUNG-1, an ongoing phase Ia/Ib trial of the HER2 TKI zongertinib (BI 1810631) in patients with advanced solid tumours with HER2 aberrations: latest data. ESMO 2023, poster 1375P
© 2023 Springer-Verlag GmbH, Impressum