EGFR-mutated NSCLC: practice-changing results and other notable findings
MARIPOSA: first-line amivantamab plus lazertinib
In the setting of EGFR-mutated NSCLC, the third-generation EGFR TKI osimertinib is the current first-line standard of care, although eventual progression is virtually inevitable. Secondary EGFR and MET alterations have been found to account for 25 % to 50 % of cases of resistance [1-3]. The global, randomized, three-arm phase III MARIPOSA trial was based on the assumption that the combination of the EGFR-MET bispecific antibody amivantamab and the third-generation EGFR TKI lazertinib, when used as a first-line strategy in locally advanced or metastatic NSCLC with EGFR mutations (i.e., exon 19 deletion or L858R mutation), might proactively address resistance and improve clinical outcomes without the addition of chemotherapy. MARIPOSA compared amivantamab plus lazertinib (n = 429; open-label) with osimertinib (n = 429; blinded) and also contained a lazertinib monotherapy arm (n = 216; blinded) that was introduced to assess the contribution of the components. Progression-free survival (PFS) with amivantamab/lazertinib vs. osimertinib by blinded independent review (BICR) constituted the primary endpoint.
Indeed, amivantamab/lazertinib, as compared to osimertinib, improved median PFS by 7.1 months, thus reducing the risk of progression or death by 30 % (23.7 vs. 16.6 months; HR, 0.70; p < 0.001) [4]. Forty-eight percent of patients in the experimental arm were progression-free at 24 months, while this was 34 % in the control arm. All of the subgroups favored the amivantamab-based approach over osimertinib. The study design of the MARIPOSA trial allowed for the estimation of extracranial PFS as serial brain MRIs were conducted on all patients. Median PFS estimates increased in both arms after censoring of CNS-only first progressions. However, a consistent benefit of the combination remained that translated into a 32 % risk reduction and PFS prolongation by 9 months (27.5 vs. 18.5 months; HR, 0.68; p < 0.001). Identical reductions in the risk of progression or death by 31 % resulted in patients with and without a history of brain metastases. At the same time, lazertinib monotherapy that was assessed in the third study arm demonstrated meaningful clinical activity. Median PFS in the lazertinib-treated group was 18.5 months, with superimposable Kaplan-Meier curves for lazertinib and osimertinib.
Data indicating long-term benefits
The confirmed objective response rates (ORRs) by BICR did not differ between amivantamab/lazertinib and osimertinib (80 % vs. 76 %), although median duration of response was improved by 9 months in the experimental arm (25.8 vs. 16.8 months). PFS2, which was PFS after the first subsequent therapy, was significantly longer in the amivantamab-treated patients, with 24-month rates of 72 % vs. 64 % (HR, 0.75; p = 0.03). While overall survival (OS) data were not mature yet, early results indicated a trend favoring amivantamab/lazertinib (HR, 0.80; p = 0.11).
In terms of safety, the combination was shown to induce higher adverse event (AE) rates related to EGFR and MET inhibition except for diarrhea, which occurred more commonly on osimertinib treatment. Most AEs were classified as grade 1 or 2. The rates of interstitial lung disease (ILD)/pneumonitis remained low, at approximately 3 % for both arms. Treatment-related AEs leading to discontinuations of all agents were observed in 10 % vs. 3 %.
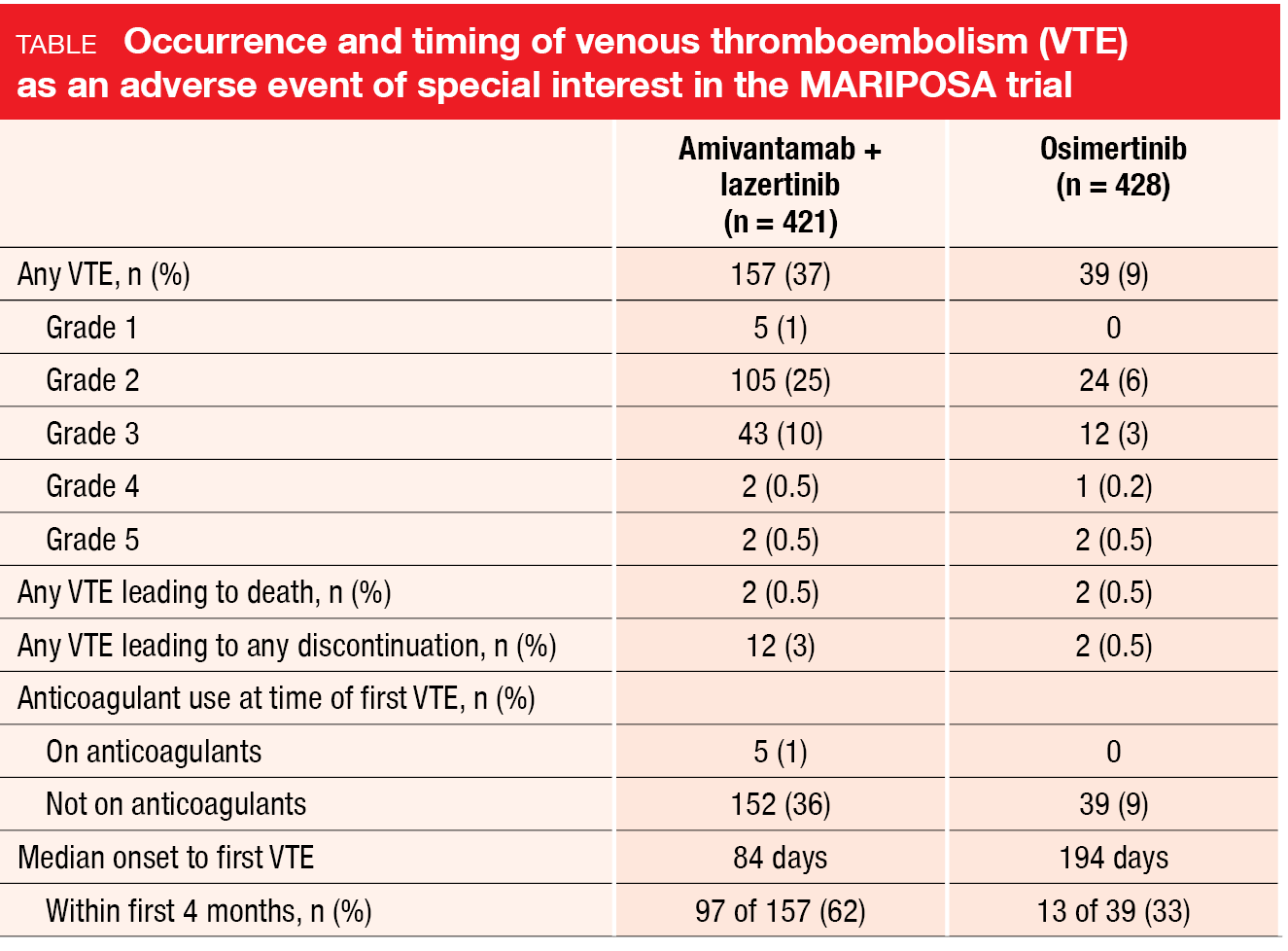
Venous thromboembolism (VTE) as an AE of special interest was noted more frequently with amivantamab/lazertinib than with osimertinib (37 % vs. 9 %; Table), although the majority of events were grade 1 or 2. Discontinuation rates due to AEs were low and comparable across arms. In most cases, the first VTE event occurring in the experimental arm was reported during the first four months of treatment, while most patients did not receive anticoagulation therapy. The authors pointed out that prophylactic anticoagulation is now recommended for the first four months of treatment in ongoing trials of amivantamab/lazertinib. In light of the findings from the MARIPOSA trial, amivantamab/lazertinib represents a new first-line standard of care in patients with EGFR-mutated advanced NSCLC.
MARIPOSA-2: amivantamab plus chemotherapy ± lazertinib
Patients who had progressed on or after osimertinib monotherapy as the most recent line in the setting of advanced EGFR-mutated NSCLC were eligible
for the global, randomized phase III MARIPOSA-2 trial. This three-arm study investigated the use of amivantamab plus chemotherapy with or without lazertinib to address osimertinib-related resistance. The patients were randomized to either amivantamab/lazertinib plus chemotherapy (n = 263), amivantamab/chemotherapy (n = 131), or chemotherapy alone (n = 263). Treated and untreated stable brain metastases were allowed. Almost half of patients showed a history of brain lesions, with 41 % to 51 % not having received brain irradiation. Serial brain MRIs were required for the total population.
MARIPOSA-2 had a dual primary endpoint of PFS by BICR: both amivantamab/lazertinib plus chemotherapy and amivantamab/chemotherapy were compared with chemotherapy only. During the study, the amivantamab/lazertinib plus chemotherapy regimen was modified to start lazertinib after carboplatin completion due to increased hematologic toxicities, and an extension cohort was established that enrolled new patients. Future analyses will explore the impact of this modification. Per protocol, the primary endpoint evaluated all randomized patients in the amivantamab/lazertinib plus chemotherapy arm irrespective of the dosing regimen administered.
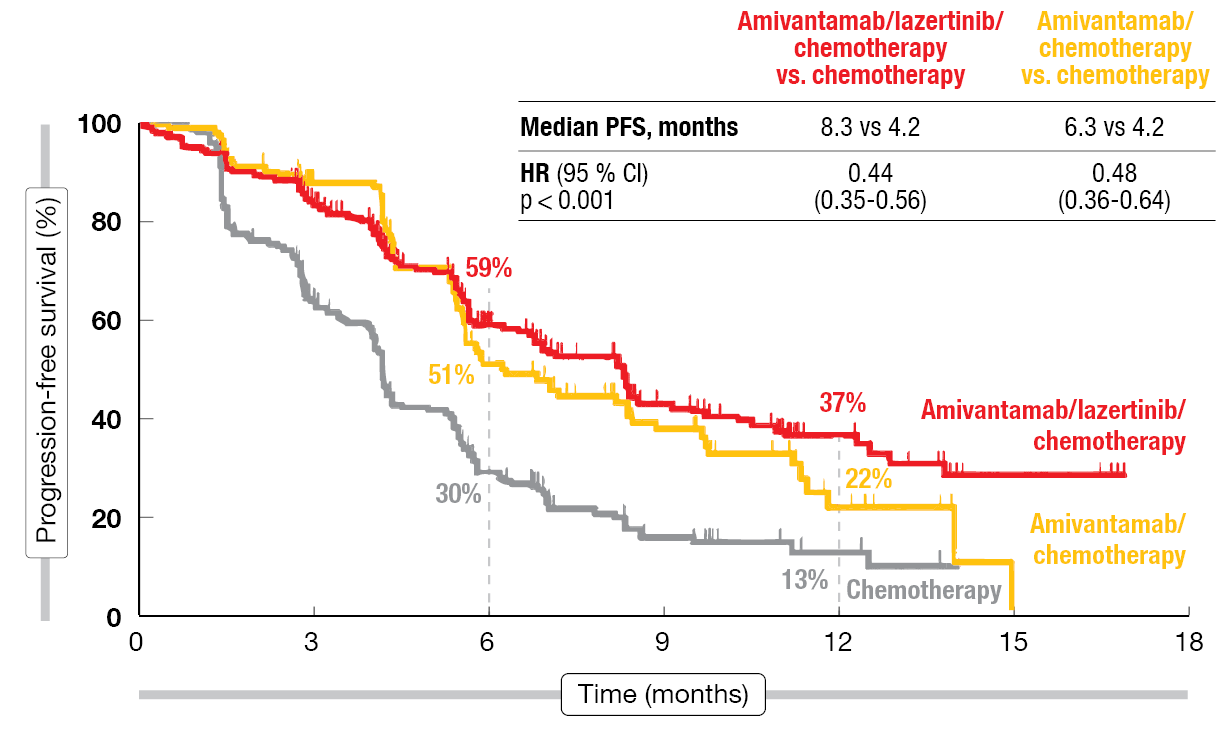
The results reported at ESMO 2023 by Passaro et al. showed that compared to chemotherapy only, both amivantamab-based regimens improved PFS [5]. At a median follow-up of 8.7 months, amivantamab/lazertinib plus chemotherapy and amivantamab/chemotherapy gave rise to reductions in the risk of progression or death by 56 % (median PFS, 8.3 vs. 4.2 months; HR, 0.44; p < 0.001) and 52 % (6.3 vs. 4.2 months; HR, 0.48; p < 0.001), respectively (Figure 1). All of the subgroups favored both regimens versus chemotherapy alone. Moreover, the ORRs were significantly higher with amivantamab/lazertinib plus chemotherapy and amivantamab/chemotherapy (63 % and 64 %, respectively) than with chemotherapy only (36 %; p < 0.001 each). Median duration of response in the experimental arms was numerically longer than in the control arm (9.4 months and 6.9 months, respectively, vs. 5.6 months).
Figure 1: MARIPOSA-2: progression-free survival with amivantamab/lazertinib plus chemotherapy and amivantamab/chemotherapy vs. chemotherapy alone
Improvement of intracranial outcomes
Likewise, both amivantamab-containing regimens diminished the risk of intracranial progression or death, with reductions of 42 % (HR, 0.58; p < 0.001) and 45 % (HR, 0.55; p = 0.001), respectively. Among patients with a history of brain metastases who had not undergone prior radiotherapy, intracranial PFS was improved by 56 % (HR, 0.44; p = 0.005) and 64 % (HR, 0.36; p = 0.013), respectively. Early data on OS indicated a trend favoring amivantamab/chemotherapy over chemotherapy alone (HR, 0.77).
Median duration of treatment was longer for the amivantamab-containing arms than for chemotherapy. However, grade ≥ 3 AEs and dose modifications were more common in these arms, particularly in the lazertinib-treated group. Treatment-related AEs necessitated discontinuations of all agents in 10 %, 8 % and 2 %, respectively. Neutropenia and thrombocytopenia mostly occurred during cycle 1, and rates of febrile neutropenia were low. VTE was most frequent with amivantamab/lazertinib plus chemotherapy (22 % vs. 10 % and 5 %), while rates of discontinuation due to VTE were low and no grade 5 events occurred. Less than 3 % of patients across all arms developed ILD.
The authors stressed that amivantamab/lazertinib plus chemotherapy and amivantamab/chemotherapy are the first regimens to demonstrate improved PFS compared to chemotherapy in EGFR-mutated advanced NSCLC after disease progression on osimertinib. Next steps include the evaluation of subcutaneous amivantamab that is expected to improve convenience and quality of life.
Osimertinib plus ramucirumab: PFS advantage in RAMOSE…
Dual inhibition of EGFR and VEGF has been identified as an intensification strategy in the setting of EGFR-mutant NSCLC. As demonstrated in the RELAY, NEJ026 and ARTemis studies, this has the potential to delay resistance and prolong PFS [6-8]. At ESMO 2023, Le et al. presented the results of the randomized phase II RAMOSE trial that explored the addition of the anti-VEGFR2 antibody ramucirumab 10 mg/kg Q3W to osimertinib 80 mg OD in 93 US-based patients with advanced EGFR-mutated (i.e., deletion 19, L858R mutation) NSCLC [9]. This combination was compared to single-agent osimertinib 80 mg OD (n = 46). The patients were treatment-naïve regarding both EGFR TKI therapy and anti-VEGF approaches. CNS metastases were present in 43.0 % and 52.0 % in the groups receiving ramucirumab plus osimertinib and osimertinib alone, respectively.
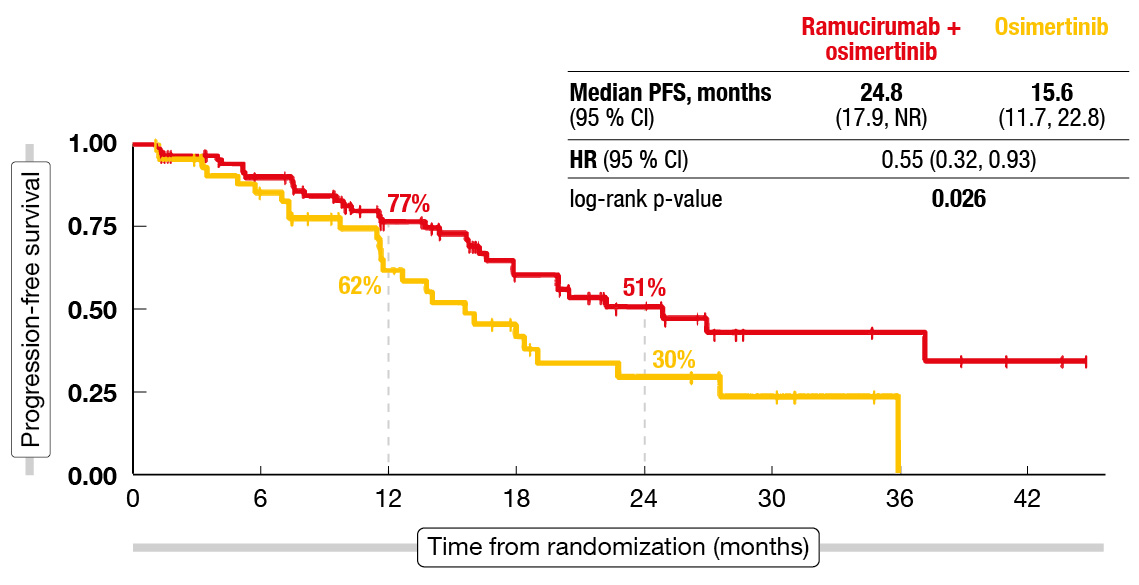
After a median follow-up of 16.6 months, the combined regimen significantly improved PFS, which was the primary endpoint, compared to osimertinib monotherapy (24.8 vs. 15.6 months; HR, 0.55; p = 0.026; Figure 2). The PFS benefit was consistent across the pre-defined subgroups that included patients with and without brain metastases. No differences were noted across the arms in terms of ORR (76.3 % vs. 80.4 %) or the disease control rate (96.8 % vs. 95.7 %). The combination regimen was well tolerated, with discontinuation rates being similarly low for both regimens (9.7 % vs. 8.7). No grade 5 events occurred, and only one grade 4 event was reported, which was hyponatremia in the combination arm. As the authors summarized, the RAMOSE trial demonstrated that the addition of ramucirumab to osimertinib can be safe and efficacious with regard to PFS in the frontline setting.
Figure 2: Primary endpoint of RAMOSE: improved progression-free survival with the addition of ramucirumab to osimertinib
… but not in OSIRAM-1
Another phase II study evaluating osimertinib plus ramucirumab in advanced untreated non-squamous NSCLC with activating EGFR mutations is the OSIRAM-1 trial. This study was conducted in Japanese patients and had the same design as RAMOSE, although with biweekly rather than triweekly administration of ramucirumab 10 mg/kg in addition to osimertinib 80 mg OD. Fifty-nine and 61 patients received the combination and osimertinib monotherapy, respectively. Individuals with asymptomatic brain metastases were eligible for enrolment.
However, OSIRAM-1 was a negative trial, with PFS by BICR showing no difference across the arms both of which performed well (20.0 vs. 24.0 months; HR, 1.054; p = 0.4621) [10]. The subgroup analysis suggested that some groups such as older patients (≥ 75 years), those with L858R mutation and those with brain metastases derived greater PFS benefit from the combination. In the cohort that had brain metastases at baseline, a 34 % risk reduction was noted in the experimental arm versus the control arm (HR, 0.655).
According to the safety analysis, the combination treatment was associated with higher rates of diminished platelet counts (all grades, 55.9 % vs. 27.4 %; grade ≥ 3, 0 % vs. 1.6 %) and neutrophil counts (all grades, 30.5 % vs. 25.8 %; grade ≥ 3, 10.2 % vs. 3.2 %), which led to early discontinuation of ramucirumab. The authors pointed out that OSIRAM-1 was conducted during the critical phase of the COVID-19 pandemic, which could have had a negative impact on the optimal administration of ramucirumab that required biweekly hospital visits. A cross-trial comparison showed that exposure to ramucirumab had been considerably longer in RAMOSE than in OSIRAM-1 (14.4 vs. 4.7 months) [11].
Ex20ins-positive NSCLC: amivantamab/chemotherapy
In the setting of advanced NSCLC with EGFR exon 20 insertion mutations (Ex20ins), the outcomes are historically poor [12-14]. Ex20ins are largely insensitive to EGFR TKIs, and checkpoint inhibitors have failed to show benefit in this setting. Platinum-based chemotherapy is usually administered, but has limited efficacy. Amivantamab has been established as a treatment option after progression on platinum-based chemotherapy. The randomized phase III PAPILLON study presented at ESMO 2023 attempted to increase the efficacy of amivantamab by combining it with carboplatin and pemetrexed in the first-line treatment of patients with locally advanced or metastatic, Ex20ins-positive NSCLC (n = 153). Chemotherapy alone was administered in the control arm (n = 155). Patients who progressed in this group were allowed to cross over to second-line amivantamab monotherapy.
PFS by BICR, which was defined as the primary endpoint, was significantly improved by the addition of amivantamab to chemotherapy, with a 60 % reduction in the risk of progression or death (11.4 vs. 6.7 months; HR, 0.395; p < 0.0001) [15]. At 18 months, 31 % vs. 3 % of patients were progression-free. All of the predefined subgroups favored the combined approach with regard to PFS.
Similarly, the ORR was significantly higher in the experimental arm (73 % vs. 47 %; OR, 3.0; p < 0.0001), and the duration of response was longer (9.7 vs. 4.4 months). Amivantamab/chemotherapy gave rise to significantly improved PFS2, i.e., PFS after the first subsequent therapy (not reached vs. 17.2 months; HR, 0.493; p = 0.001), with 24-month PFS2 rates of 57 % vs. 35 %. This finding supports the first-line use of the combination. Sixty-six percent of patients in the control arm whose disease progressed crossed over to amivantamab. Regarding interim OS, amivantamab/chemotherapy showed a trend with a 32 % reduction in mortality (not reached vs. 24.4 months; HR, 0.675; p = 0.106).
The safety profile of amivantamab plus chemotherapy was consistent with the profiles of the individual agents. Across the arms, the rates of discontinuation of all study agents due to AEs were similar. For amivantamab, the analysis showed a low discontinuation rate of 7 % due to treatment-related events. Pneumonitis developed in 4 (3 %) patients in the combination arm. EGFR- and MET-related AEs were increased in the experimental arm, although these were mainly grade 1 or 2. Chemotherapy-associated gastrointestinal and hematologic AEs were comparable except for neutropenia that occurred more frequently with the combination (all grades, 59 % vs. 45 %; grade ≥ 3, 33 % vs. 23 %), although it was transient and led to low rates of discontinuation. The authors concluded that amivantamab plus chemotherapy represents the new standard of care for the first-line treatment of patients with advanced NSCLC harboring EGFR exon 20 insertion mutations.
Afatinib in uncommon EGFR mutations
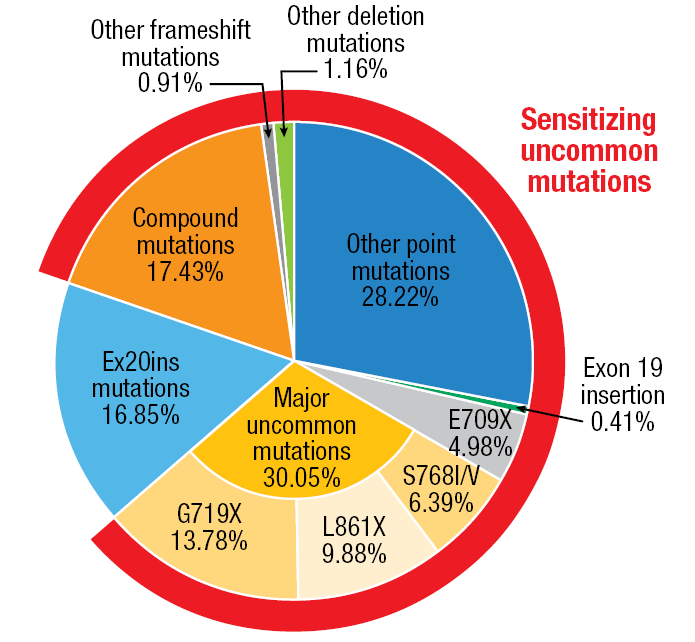
To date, there has been a lack of randomized phase III trial in patients harboring sensitizing uncommon mutations defined as uncommon/compound EGFR mutations without Ex20ins and de-novo T790M mutation (Figure 3). Therefore, the randomized phase III ACHILLES/TORG1834 trial was initiated to compare the second-generation EGFR TKI afatinib 40 mg or 30 mg OD (n = 73) with platinum plus pemetrexed (n = 36) as first-line treatment of locally advanced or metastatic non-squamous NSCLC harboring sensitizing uncommon mutations. At ESMO 2023, Miura et al. reported the first results of the ACHILLES/TORG1834 study [16].
Afatinib significantly improved PFS compared to platinum doublet chemotherapy. After a median follow-up of 12.5 months, treatment with the EGFR TKI led to a 58 % risk reduction (median PFS, 10.6 vs. 5.7 months; HR, 0.422; p = 0.0007). Thus, the study met its primary endpoint. The 12-month PFS rates were 42.1 % vs. 19.3 %. All of the subgroups derived PFS benefit from afatinib treatment compared to chemotherapy. The ORRs did not differ significantly across the treatment arms (61.4 % vs. 47.1 %; p = 0.2069).
No new safety signals were reported for afatinib compared to previous reports; diarrhea, paronychia, rash and mucositis occurred as the most common AEs. In their summary, the authors concluded that ACHILLES/TORG1834 confirmed afatinib as the standard of care for patients with treatment-naïve non-squamous NSCLC harboring sensitizing uncommon EGFR mutations.
Figure 3: Sensitizing uncommon EGFR mutations: uncommon/compound mutations without exon 20 insertion and de-novo T790M mutation
REFERENCES
- Moores SL et al., A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res 2016; 76(13): 3942-3953
- Vijayaraghavan S et al., Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther 2020; 19(10): 2044-2056
- Yun J et al., Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov 2020; 10(8): 1194-1209
- Cho BC et al., Amivantamab plus lazertinib versus osimertinib as first-line treatment in EGFR-mutated advanced NSCLC. ESMO 2023, abstract LBA14
- Passaro A et al., Amivantamab plus chemotherapy (with or without lazertinib) vs. chemotherapy in EGFR-mutated, advanced NSCLC after progression on osimertinib. ESMO 2023, abstract LBA15
- Nagakawa K et al., Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY):
a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(12): 1655-1669 - Saito et al., Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019; 20(5): 625-635
- Zhou Q et al., Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509):
A multicenter phase 3 study. Cancer Cell 2021; 39(9): 1279-1291 - Le X et al., A multi-centre open-label randomized phase II study of osimertinib with and without ramucirumab in TKI-naïve EGFR-mutant metastatic NSCLC (RAMOSE trial). ESMO 2023, abstract LBA71
- Nakahara Y et al., OSIRAM-1: A multicenter, open label, randomized phase II study of osimertinib plus ramucirumab versus osimertinib alone as initial chemotherapy for EGFR mutation-positive non-squamous non-small cell lung cancer (TORG1833). ESMO 2023, LBA70
- Wu YL, New wine in old bottles, Discussion for LBA70 & LBA71, ESMO 2023, 21st October, 2023, Madrid, Spain
- Bazhenova L et al., Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer 2021; 162: 154-161
- Ou S-H et al., Real-world response and outcomes in patients with NSCLC with EGFR exon 20 insertion mutations. JTO Clin Res 2023; 4(10): 100558
- Chouaid C et al., A real-world study of patients with advanced non-squamous non-small cell lung cancer with EGFR exon 20 insertion: Clinical characteristics and outcomes. Target Oncol 2021; 16(6): 801-811
- Girard N et al., Amivantamab plus chemotherapy vs chemotherapy as first-line treatment in EGFR exon 20 insertion-mutated advanced non-small cell lung cancer. ESMO 2023, abstract LBA5
- Miura S et al., Afatinib versus chemotherapy for treatment-naïve non-small cell lung cancer with a sensitizing uncommon epidermal growth factor receptor mutation: a phase III study. ESMO 2023, abstract LBA66
© 2023 Springer-Verlag GmbH, Impressum