Small-cell lung cancer: insights and new treatment options centering around DLL3
Target with prognostic and predictive value
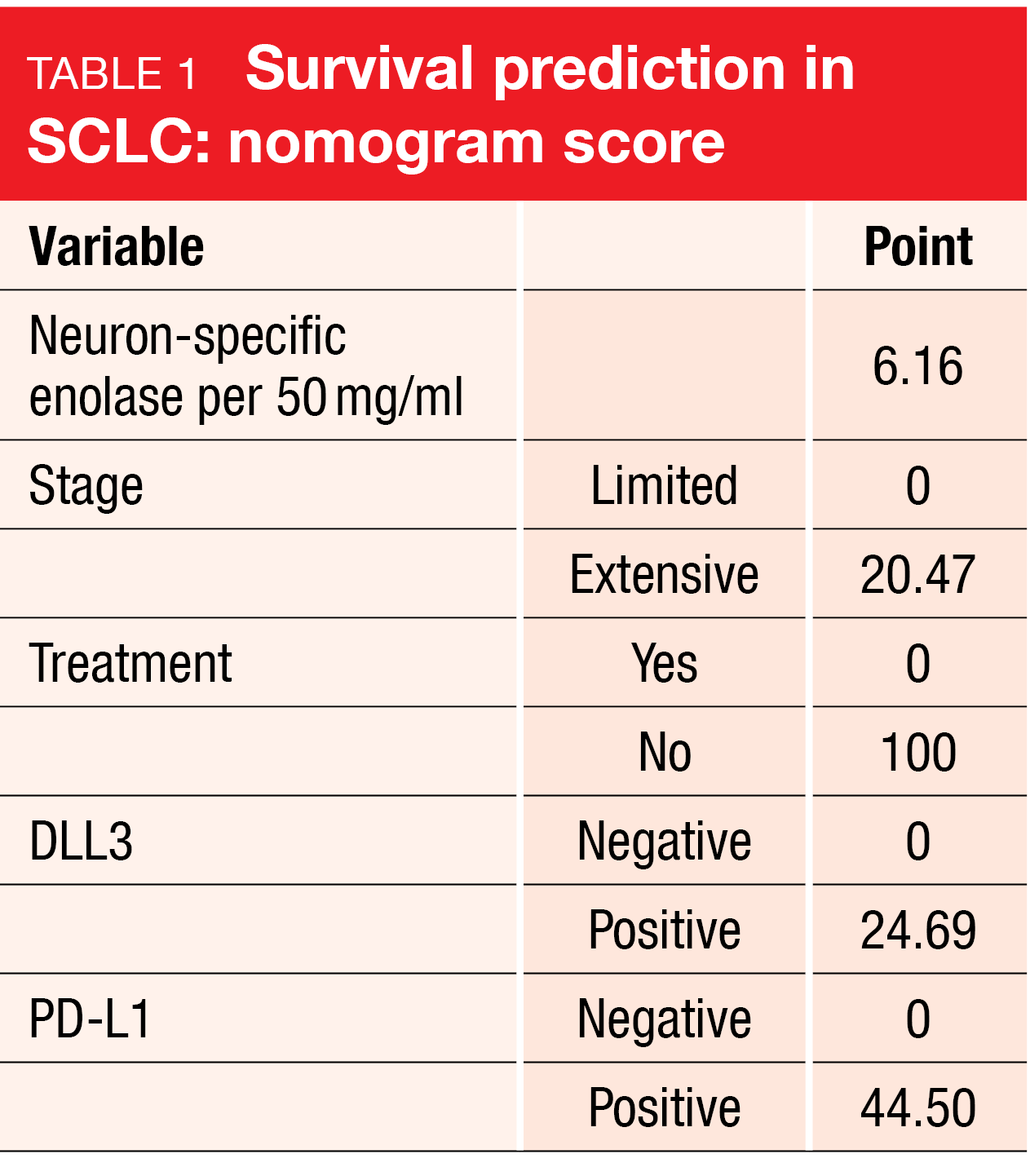
The cell surface protein delta-like-ligand 3 (DLL3) is an emerging therapeutic target in neuroendocrine tumors and neuroendocrine carcinomas such as small-cell lung cancer (SCLC). Approximately 75 % of SCLCs express DLL3 [1]. Data reported at ESMO 2023 showed that high DLL3 expression is associated with poor overall survival, advanced pathological grade, and a distinct immune landscape across neuroendocrine neoplasms found in the lung, prostate, and bladder [2]. A Chinese study group developed a prediction model for the 12-month survival probability of patients with SCLC based on the expression of DLL3 and PD-L1 and other factors (Table 1) that showed excellent performance and might assist physicians in clinical decision making [3].
DLL3-targeted agents such as the bispecific DLL3/CD3 T-cell engager BI 764532 are currently under clinical development. BI 764532 binds to both DLL3 on cancer cells and CD3 on the surface of T-cells, which leads to T-cell activation and cancer cell apoptosis [4]. In an ongoing phase I dose escalation and expansion study, BI 764532 has induced tumor shrinkage at clinically active doses in patients with SCLC and other neuroendocrine carcinomas [5]. Based on the observation that BI 764532 upregulates the PD-L1 pathway, the combination of the T-cell engager with the PD-1 inhibitor ezabenlimab will be evaluated in a phase I dose escalation trial in the setting of DLL3-positive SCLC and other neuroendocrine neoplasms expressing DLL3 (NCT05879978) [6]. The patients participating in this trial have failed available standard therapies or are not eligible for them.
DeLLphi-301: tarlatamab at two doses
Another bispecific DLL3/CD3 T-cell engager is tarlatamab that was investigated in the open-label phase II DeLLphi-301 study in patients with extensive-stage SCLC who had previously received ≥ 2 treatment lines including platinum-doublet therapy. In the dose evaluation part of the study (part 1), 88 patients each received either tarlatamab 10 mg or 100 mg in a randomized manner. The treatment started with a 1 mg dose on day 1 that was followed by either 10 or 100 mg on days 8, 15 and Q2W thereafter. The 10 mg dose was selected for further assessment. In the dose expansion part (part 2), 12 patients received tarlatamab 10 mg according to the same dosing schedule. Part 3 of the study entailed reduced inpatient monitoring; here, 34 patients were treated with tarlatamab 10 mg. DLL3 expression was no prerequisite for study entry. Treated and stable brain metastases were permitted.
The objective response rate (ORR) constituted the primary endpoint, as well as treatment-emergent adverse events (TEAEs) and tarlatamab serum concentrations. At ESMO 2023, Paz-Ares et al. presented the primary analysis of the DeLLphi-301 trial [7]. Across parts 1 and 2, 100 patients had received tarlatamab 10 mg, while 88 had been treated with 100 mg in part 1. In these two groups, 33 % and 43 %, respectively, had received ≥ 3 prior lines of therapy, with anti-PD-(L)1 treatment having been administered in 73 % and 70 %, respectively. DLL3 expression was present in 96 % in each group.
Durable antitumor activity
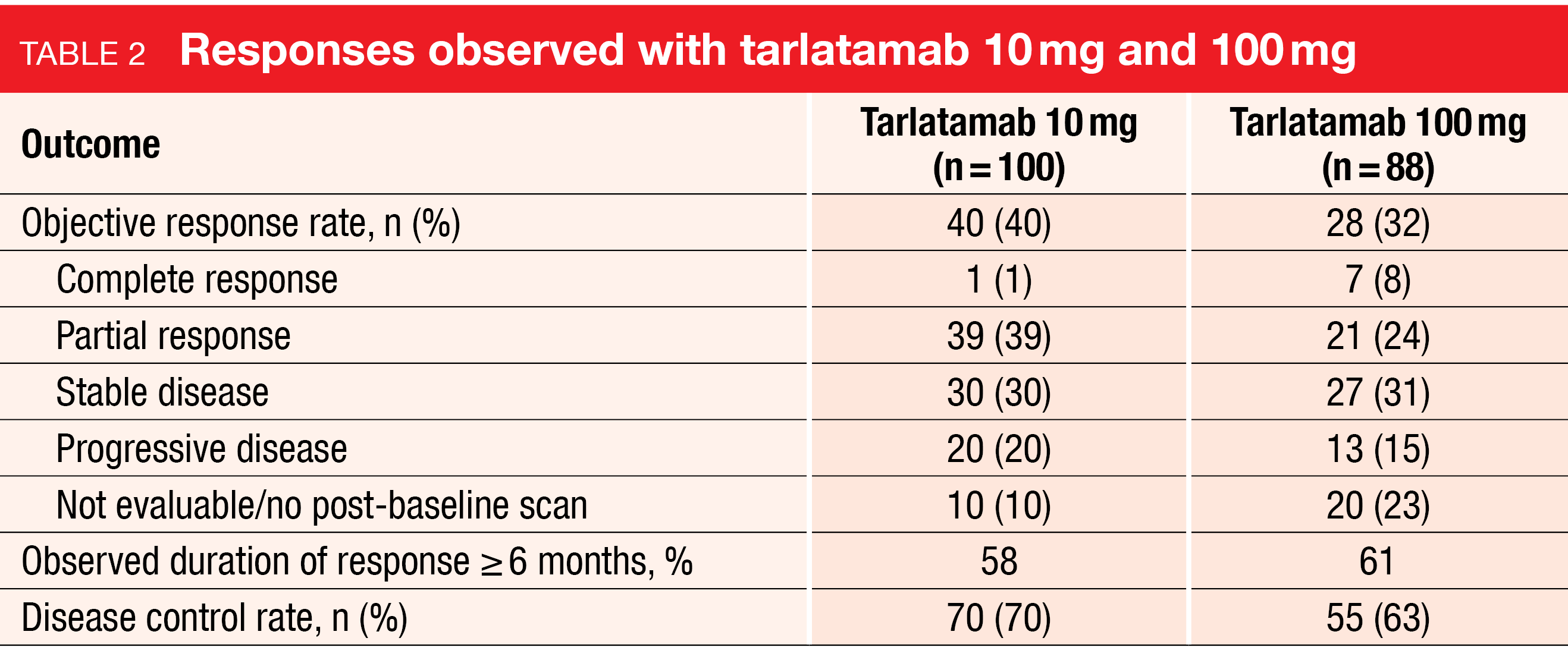
In this heavily pretreated patient population, tarlatamab 10 mg demonstrated clinical efficacy with an ORR of 40 % and a disease control rate (DCR) of 70 % (Table 2). Tarlatamab 100 mg gave rise to an ORR of 32 % and a DCR of 63 %. Responses occurred regardless of DLL3 expression and were also observed in patients whose tissue samples were not evaluable for DLL3 expression. Among 68 responders (n = 40 and 28 with 10 mg and 100 mg, respectively), 59 % showed duration of response of ≥ 6 months. Median duration of response had not been reached at the time of the analysis. Median progression-free survival was 4.9 and 3.9 months for tarlatamab 10 mg and 100 mg, respectively. At 6 months, 40.4 % and 34.1 % of patients, respectively, were progression-free. Data were not mature for overall survival (OS). Median OS was 14.3 months for the 10 mg dose and had not been reached for the 100 mg dose. The 6-month OS rates were 73.4 % and 71.4 %, respectively. At data cutoff, 57 % and 51 % of patients in the 10 mg and 100 mg groups, respectively, were alive.
Tarlatamab demonstrated a favorable safety profile. Cytokine release syndrome (CRS) represented the most common TEAE, with rates of 49 %, 61 % and 56 % for tarlatamab 10 mg (part 1 and 2), 100 mg, and 10 mg (part 3), respectively. CRS events were generally confined to the first or second dose, classified as grade 1 or 2, and manageable with supportive care. Other AEs included decreased appetite, pyrexia, constipation, and anemia. Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred infrequently and was predominantly observed with the 100 mg dose.
Dose interruptions or reductions due to TEAEs occurred in 14 %, 29 % and 9 % with tarlatamab 10 mg (part 1 and 2), 100 mg, and 10 mg (part 3), respectively. The discontinuation rates due to treatment-related AEs were low at 4 %, 3 % and 0 %, respectively. Shorter inpatient monitoring as performed in part 3 did not alter the safety profile. In their conclusion, the authors emphasized that these results support the use of tarlatamab in patients with pretreated SCLC. The ongoing phase III DeLLphi-304 study will compare tarlatamab 10 mg Q2W with standard-of-care chemotherapy in the setting of relapsed SCLC (NCT05740566).
Recruitment halt in TREASURE
The randomized TREASURE trial was initiated based on the hypothesis that consolidation thoracic irradiation in addition to atezolizumab maintenance after induction therapy consisting of 4 cycles of carboplatin/etoposide plus atezolizumab might improve OS in extensive-stage SCLC. Patients in arm A of the TREASURE study underwent thoracic radiotherapy with 30 Gy over 10 days plus atezolizumab maintenance, while those in arm B received atezolizumab maintenance only. OS and toxicity (pneumonitis grade ≥ 3) were defined as the primary endpoints.
However, unexpected safety signals necessitated a recruitment halt. At ESMO 2023, Bozorgmehr et al. reported that during a routine safety review by the Safety Monitoring Committee, a potential imbalance in the total number of fatal AEs was identified, with 5 vs. 1 cases in arms A vs. B [8]. An unplanned interim OS analysis was conducted and recruitment was stopped. A re-analysis performed three months later showed no change in OS but a three times higher number of severe AEs in the intervention arm (28 vs. 9), along with a further increase in fatal AEs (6 vs. 1).
The causes of death in the experimental arm were diverse and not clearly tied to either immunotherapy or irradiation. They included two cases of sepsis and one case each of multi-organ failure, lung infection, pneumonitis, and worsening of the patient’s general condition. In the control arm, one patient died due to hepatic failure. An in-depth serious AE analysis on a case-to-case basis did not reveal any common thread. Nevertheless, it was decided to permanently halt recruitment due to the imbalance in number, severity and seriousness of severe AEs. Factors leading to this unexpected outcome remain to be identified. The authors expressed hope that the final analysis including radiotherapy and biomarker parameters might contribute to further insights.
REFERENCES
- Huang RSP et al., Delta-like protein 3 prevalence in small cell lung cancer and DLL3 (SP347) assay characteristics. Arch Pathol Lab Med 2019; 143(11): 1373-1377
- Crymes A et al., Landscape of Delta-like-ligand 3 (DLL3) expression across neuroendocrine neoplasms. ESMO 2023, poster 1188P
- Zhao J et al., Novel nomogram based on the expression of DLL3 and PD-L1 for predicting the prognosis of small cell lung cancer patients. ESMO 2023, poster 2018P
- Wermke M et al., Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Future Oncol 2022; 18(24): 2639-2649
- Wermke M et al., First-in-human dose-escalation trial of BI 764532, a delta-like ligand 3 (DLL3)/CD3 IgG-like T-cell engager in patients with DLL3-positive small-cell lung cancer and neuroendocrine carcinoma. J Clin Oncol 2023; 41(suppl 16; abstr 8502)
- Mazières J et al., Phase I, non-randomised, open-label, multi-centre dose escalation trial of BI 764532 (DLL3/CD3 IgG-like T-cell engager) + ezabenlimab (anti-PD-1 antibody) in patients with small cell lung cancer and other neuroendocrine carcinomas expressing DLL3. ESMO 2023, poster 2028TiP
- Paz-Ares L et al., Tarlatamab for patients with previously treated small cell lung cancer: Primary analysis of the phase 2 DeLLphi-301 study. ESMO 2023, abstract LBA92
- Bozorgmehr F et al., Recruitment discontinuation in TREASURE trial (Thoracic RadiothErapy with Atezolizumab in Small cell lUng cancer extensive disease) due to unexpected safety data. ESMO 2023, abstract 1988MO
© 2023 Springer-Verlag GmbH, Impressum