Exploring chemotherapy-free approaches in the treatment of DLBCL
Smart Stop: quadruplet therapy
For decades, the CHOP regimen has been the first-line standard of care for patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL). Significant improvement was achieved through the addition of rituximab (R-CHOP). Ever since, however, multiple clinical trials investigating expanded or alternative treatment regimens have not succeeded in further improving patient outcomes. The Smart Start study assessing rituximab, lenalidomide and ibrutinib was the first study to address newly diagnosed DLBCL with a targeted combination before chemotherapy [1]. This regimen gave rise to an overall response rate (ORR) of 86 %, a complete remission (CR) rate of 36 % and durable responses, thus establishing the potential for the development of biologically driven and non-cytotoxic first-line therapies in the setting of DLBCL.
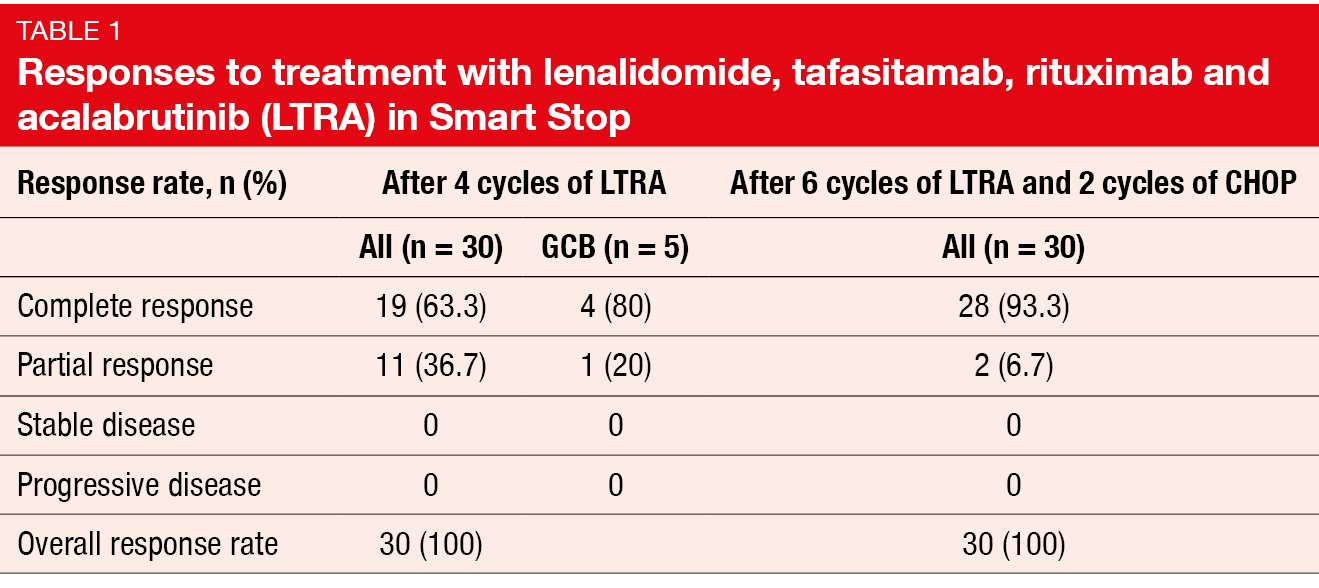
In order to improve upon Smart Start, the single-center, open-label phase II Smart Stop study was conducted using the LTRA combination, i.e., lenalidomide, the CD19-directed antibody tafasitamab, rituximab, and the second-generation BTK inhibitor acalabrutinib instead of ibrutinib. At ASH 2023, Westin et al. reported the results for Cohort 1 (n = 30) [2]. After 4 cycles of LTRA, patients who showed CR according to PET/CT received 6 cycles of LTRA, with CHOP being administered for the first 2 cycles only. Those who had partial response (PR), stable disease (SD) or disease progression after the initial 4 cycles were treated with 6 cycles of LTRA plus CHOP. The ORR after 4 cycles of LTRA and the CR rate at the end of therapy constituted the primary endpoints.
This comparison was based on the hypothesis that LTRA for 4 cycles will improve upon the 36 % CR observed in Smart Start, thus enabling the use of less or no chemotherapy. Patients with any subtype of large B-cell lymphoma were eligible. Twenty-five (83 %) had a non-germinal center B-cell (GCB) subtype, while 5 (17 %) had a GCB subtype.
Deep responses despite reduced CHOP
After 4 cycles of LTRA, the CR rate was 63.3 %, which included four of the five GCB patients (Table 1). All patients responded to the treatment. Nineteen patients achieved CR and were allocated to 2 cycles of CHOP plus 6 cycles of LTRA, while 11 concurrently received LTRA and CHOP for 6 cycles after failing to achieve CR. The CR of the total group after 6 cycles of LTRA and 2 cycles of CHOP was 93.3 % (Table 1). At the time of the analysis, 22 individuals had completed the entire treatment; all of them achieved CR. Seven of the first 10 patients received only 2 cycles of CHOP and remained in ongoing remission at > 9 months. To date, no patient has progressed or died.
The depth of response was assessed using circulating tumor DNA (ctDNA) in 15 patients. Among these, 12 (80 %) achieved CR according to imaging. Five patients (33 %) had undetectable disease burden after 4 cycles of LTRA. In 87 %, a > 2 log-fold reduction was observed. Toxicities of interest reported with LTRA and CHOP included neutropenia (87 %), fatigue (73 %), rash (43 %) and infections (30 %). Rash was manageable with dose reductions and interruptions. Neutropenia was the most common toxicity with the combined regimen (any grade, 80 %) but did not lead to dropouts due to infections.
In their summary, the authors noted that targeted therapies alone are safe and effective as initial first-line treatment in patients with DLBCL, with less than 6 cycles of CHOP appearing feasible in case of response to LTRA with short follow-up. Basically, the Smart Stop study established the feasibility of targeted therapy with reduced chemotherapy and of response-adapted trials evaluating novel combinations. The next stage of the Smart Stop study will assess the efficacy of a completely chemotherapy-free approach involving 6 further cycles of LTRA in patients who have achieved CR after the first 4 cycles.
Mosunetuzumab/polatuzumab vedotin for the unfit
In elderly, unfit or frail patients, first-line standard treatment with R-CHOP is frequently administered at reduced doses such as R-miniCHOP [3], although attenuated regimens are associated with inferior outcomes [4]. This raises the need for approaches that are both less toxic and more efficacious. At ASH 2023, Olszewski et al. presented initial safety and efficacy data from an ongoing phase I/II study evaluating the combination of the CD20xCD3 bispecific antibody mosunetuzumab and the CD79b-directed antibody-drug conjugate polatuzumab vedotin as first-line therapy for elderly and unfit patients (i.e., aged ≥ 80 years or 65–79 years and considered ineligible for chemoimmunotherapy) [5]. Step-up dosing of subcutaneously administered mosunetuzumab in cycle 1 was followed by mosunetuzumab 45 mg on day 1 of cycles 2–8. Patients who achieved PR or SD at the end of this treatment period were allowed to continue for up to 9 additional cycles. Polatuzumab vedotin was administered intravenously on day 1 of cycles 1–6.
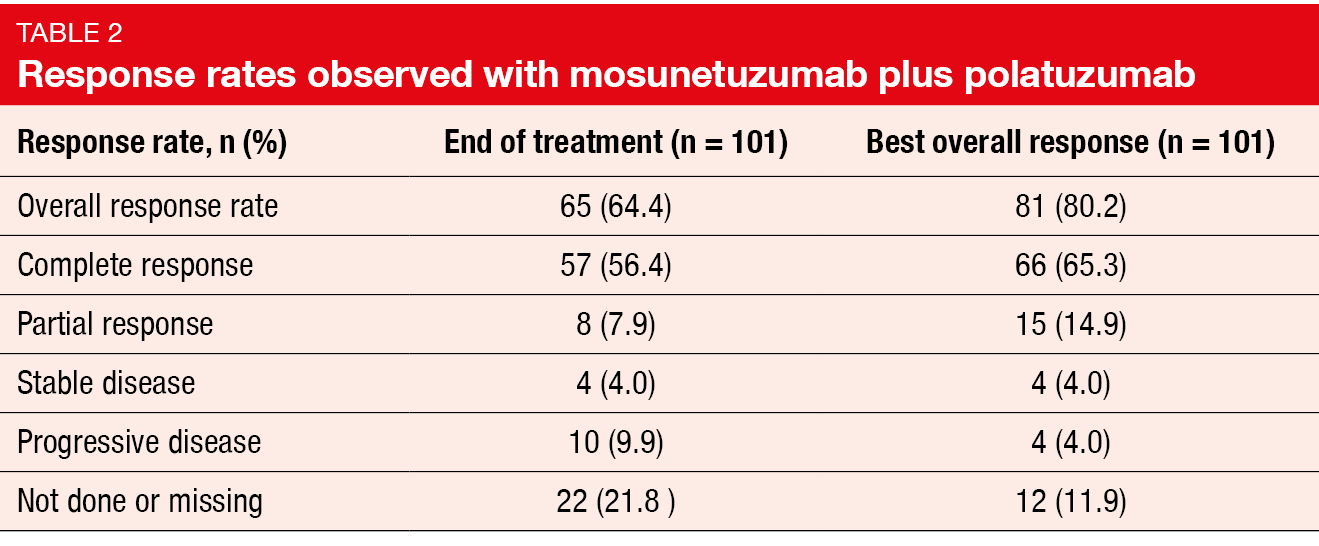
The ORR at the time of the primary response assessment after cycle 8 was defined as the primary efficacy endpoint. Other cytokine release syndrome (CRS) mitigation strategies in addition to step-up dosing of mosunetuzumab included pre-medication with dexamethasone, acetaminophen and diphenhydramine. According to the simplified geriatric assessment, only one out of 108 patients (0.9 %) was considered fit; 59.3 % and 39.8 % were classified as unfit and frail, respectively. Multiple comorbidities and polypharmacy were present. The safety and efficacy cohorts comprised 108 and 101 patients, respectively.
Mosunetuzumab/polatuzumab vedotin was shown to induce encouraging response rates and durable CRs. At the end of treatment, the ORR was 64.4 %, and 56.4 % of patients experienced CR (Table 2). The best ORR was 80.2 %, with CRs resulting in 65.3 %. The difference between these two analyses is attributed to 22 patients who were not evaluable at the end of treatment due to adverse events (AEs), death, and subject withdrawal after attaining response. This again reflects the frailty and high comorbidity burden of this study population. Six of 8 patients with PR at the end of treatment continued mosunetuzumab therapy beyond cycle 8, and three of them succeeded in converting to CR. Median duration of CR had not been reached, and 71.4 % remained in remission at 9 months. Similar observations were made with respect to progression-free survival (PFS). Median PFS was 11.9 months; at 12 months, 49.7 % of patients were progression-free. Overall, 63.4 % of patients remained in follow-up without any evidence of progression.
Fatal COVID-19 infections mainly in frail patients
Immunohistochemistry and immunofluorescence analyses based on pre-treatment biopsies found no association between the tumor immune cell composition and the treatment response, as the latter was independent of the baseline levels of B cells, cytotoxic T cells, and activated lymphocytes. This suggests that combining a bispecific antibody with an antibody-drug conjugate contributes to mitigating the dependence of responses on these features.
Overall, the safety profile of the combination was consistent with that of the individual drugs. Among mosunetuzumab-related AEs, neutropenia (any grade, 36.1 %) and CRS (any grade, 29.6 %) prevailed. CRS events were mostly grade 1 and 2 and were well managed, with no treatment discontinuations reported due to this AE. Grade 3/4 neutropenia occurred in 30.6 %, although no febrile neutropenia was observed. Serious infections were noted in 25.0 % (grade ≥ 3, 23.1 %). Thirteen of 18 fatal AEs were infections, and 10 (77 %) of these were COVID-19 infections that mostly affected patients who were frail per simplified geriatric assessment. Eighty percent of fatal COVID-19 events occurred during the Omicron waves in 2022. All patients had received at least one dose of COVID-19 vaccine, and seven had undergone COVID-19–specific antiviral treatments.
The authors noted that the number of fatal AEs other than COVID-19 was comparable with those observed in similar patient populations with DLBCL. They concluded that elderly unfit or frail patients with previously untreated DLBCL may be at increased risk of severe complications of COVID-19 infections. At the same time, regimens such as mosunetuzumab/polatuzumab vedotin might help to improve the future treatment landscape for this understudied population. Incorporation of simplified geriatric assessments in clinical trials of elderly unfit or frail patients appeared feasible and beneficial to identify a population for treatment approaches that minimize or eliminate cytotoxic chemotherapy.
Zanubrutinib, rituximab & polatuzumab vedotin
A Chinese study investigated the combination of polatuzumab vedotin, zanubrutinib and rituximab in previously untreated frail and elderly patients (aged > 70 years or 60–69 plus ECOG 2–4) with DLBCL. After 3 cycles of triplet therapy, patients who had CR or PR continued treatment for another 3 cycles, while those with SD or disease progression received second-line agents. The second response assessment at cycle 6 was followed by zanubrutinib monotherapy in case of CR, whereas patients with PR, SD or progressive disease went on to second-line treatment. Polatuzumab vedotin and rituximab were administered intravenously on day 1 of each cycle, while zanubrutinib 160 mg BID was taken on days 1-21 of the 21-day cycles. The ORR after 6 cycles constituted the primary endpoint. At ASH 2023, Ren et al. reported findings for 19 patients, most of whom were aged ≥ 70 years, had IPI scores of 3–5, and Ann Arbor disease stage III-IV [6].
All of the 12 patients who underwent the interim efficacy assessment after 3 cycles responded, with 8 and 4 obtaining CR and PR, respectively. Three patients were evaluable for the primary endpoint; in this group, too, all patients were responders, and all of them achieved CR. After a median follow-up of 114 days, no progression or relapse had occurred. The most common any-grade AEs were decreased neutrophil count (15.8 %) and upper respiratory infection (10.5 %). Lung infections represented the most common grade 3/4 AE. All patients recovered from AEs under best supportive care. The zanubrutinib dose was decreased to 80 mg BID in one individual due to transaminase elevation.
In their summary, the authors noted that the triple combination of polatuzumab vedotin, rituximab and zanubrutinib showed promising efficacy with rapid and deep responses in previously untreated frail and elderly patients with DLBCL. A prospective phase IIb trial is ongoing to further evaluate the triplet regimen as first-line treatment for this population (NCT05940064).
ELM-2: odronextamab
Patient outcomes in the relapsed/refractory DLBCL setting are still poor, and there is an unmet need for novel therapies that provide rapid disease control while improving long-term outcomes. The CD20xCD30 bispecific antibody odronextamab has shown encouraging efficacy and generally manageable safety in heavily pretreated patients with relapsed/refractory DLBCL in the phase I ELM-1 study [7] and the open-label, multicohort, multicenter phase II ELM-2 trial. At the time of the interim analysis of ELM-2, the ORR was 49.2 %, with a CR rate of 30.8 % and median duration of response of 10.2 months [8]. Ayyapan et al. presented the final analysis of the relapsed/refractory DLBCL cohort from the ELM-2 study at ASH 2023 [9].
Patients in this cohort (n = 127) were refractory to or had relapsed after ≥ 2 prior lines of therapy, which included an anti-CD20 antibody and an alkylator. Overall, this was a heavily pretreated, highly refractory population. Fifty-five percent had primary refractory disease, and 86.6 % were refractory to the last line of therapy. Cycle 1 included the step-up to odronextamab 20 mg, while odronextamab 160 mg was administered on days 1, 8 and 15 of cycles 2–4. Finally, maintenance treatment consisted of 320 mg Q2W from cycle 5. Patients who achieved CR for ≥ 9 months in the maintenance phase could transition from biweekly to four-weekly intervals. ORR by independent review was defined as the primary endpoint.
Encouraging efficacy in high-risk individuals
After a median follow-up of 29.9 months, 52.0 % of patients responded, and 31.5 % achieved CR. The median duration of response and CR was 10.2 and 17.9 months, respectively. At 12 and 24 months, CRs persisted in 61.5 % and 47.2 % of patients, respectively. Odronextamab gave rise to encouraging ORRs across high-risk subgroups such as elderly patients and those with transformed and double-hit disease. In the total study group, median PFS was 4.4 months, with 12-month and 24-month PFS rates of 29.6 % and 21.1 %, respectively. Patients who obtained CR fared considerably better with respect to median PFS than those achieving PR (20.4 and 5.8 months, respectively). At 24 months, 47.5 % vs. 18.9 % of complete and partial responders, respectively, were progression-free. Likewise, median OS had not been reached in patients with CR and was 17.0 months in those with PR. In the overall population, median OS was 9.2 months.
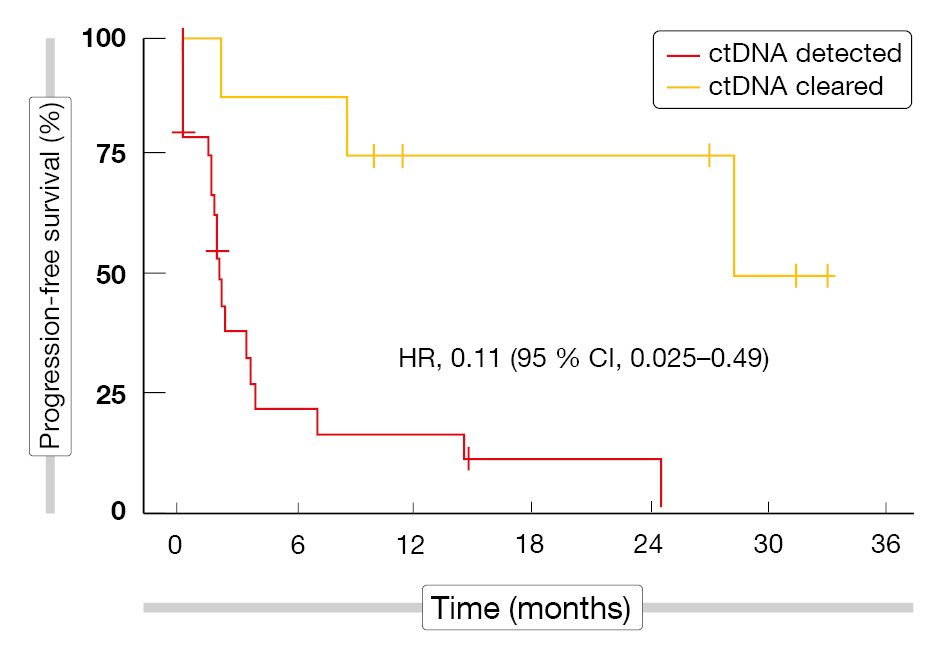
Exploratory biomarker analyses demonstrated that PFS was better in patients with undetectable minimal residual disease (MRD) than in those with ctDNA that persisted at day 15 of cycle 4 (HR, 0.27). Even in the absence of CR, there was a marked difference between the cohorts with detectable and cleared ctDNA (Figure 1). Moreover, the assessment of PFS by the LymphGen classification indicated that the EZB subtype conferred longer PFS than the other subtypes.
The safety profile of odronextamab therapy was consistent with previous reports. CRS was observed in 55.1 %, although most events were grade 1 (40 %) or 2 (11.7 %) and confined to cycle 1. With supportive measures, they resolved within a median of 2 days. No immune effector cell-associated neurotoxicity syndrome (ICANS) events were reported. Pyrexia occurred in 22.8 % and neutropenia in 20.5 %. Treatment-related AEs led to dose reduction and discontinuation in 3.1 % and 9.4 %, respectively. Any-grade infections emerged in 64.6 %, with COVID-19 observed in 18.1 % that was fatal in 5 patients (3.9 %). Other infection events included pneumonia (14.2 %), upper respiratory tract infection (8.7 %), urinary tract infection (8.7 %), and Pneumocystic jirovecii pneumonia (6.3 %). Treatment-related infections prompted permanent discontinuation of odronextamab in 4.7 %. Patient-reported outcomes were collected using the EORTC QLQ-C30 and other instruments throughout the study. According to this analysis, the scores for pain and emotional functioning were maintained or improved from baseline through week 42.
Taken together, the final results from the pivotal phase II ELM-2 study confirm highly encouraging clinical activity of odronextamab in patients with heavily pretreated relapsed/refractory DLBCL. Based on these findings, the randomized phase III trials OLYMPIA-3 and OLYMPIA-4 are evaluating odronextamab in patients with previously untreated and relapsed/refractory DLBCL, respectively.
Figure 1: Progression-free survival by ctDNA MRD response in patients without complete remission at day 15 of cycle 4 of odronextamab therapy
Epcoritamab plus lenalidomide
Avivi et al. reported the first results for the combination of the subcutaneously administered CD3xCD20 bispecific antibody epcoritamab and lenalidomide in patients with CD20-positive, relapsed/refractory DLBCL who were treated in arm 1 of the EPCORE NHL-5 study (n = 35) [10]. They had previously received ≥ 1 anti-CD20 antibody-containing systemic therapy and were ineligible for autologous stem cell transplantation or had failed prior stem cell transplantation; prior CAR T-cell therapy was allowed. This was a high-risk population, as 51 % had R-IPI scores of 3–5 and primary refractory disease was present in 43 %. Prior CAR T-cell therapy had been administered in 23 %. The experimental treatment in arm 1 of the EPCORE NHL-5 trial included twelve 28-day cycles of the combination. Epcoritamab was administered at its full dose of 48 mg on days 15 and 22 of cycle 1 after initial step-up, as well as on days 1, 8, 15 and 22 of cycles 2–3 and on day 1 of cycles 4–12. Lenalidomide 25 mg OD was taken on days 1–21 of all 12 cycles. CRS prophylaxis using diphenhydramine, acetaminophen and corticosteroids was mandatory with the first four epcoritamab doses.
Epcoritamab in combination with lenalidomide induced deep and durable responses. The ORR was 71.9 %, with CR resulting in 53.1 %. ORR and CR rates were fairly consistent across the pre-specified high-risk subgroups including patients with advanced age and prior CAR T-cell therapy. Median duration of CR had not been reached at the time of the analysis. MRD negativity was achieved early on, with 83 % of patients showing MRD-negative CR after 2 cycles, and was sustained throughout treatment.
Epcoritamab plus lenalidomide demonstrated a manageable safety profile that was consistent with the established profiles. Grade 3/4 epcoritamab-related treatment-emergent AEs (TEAEs) were observed in 66 %. TEAE-induced delay or interruption of epcoritamab was observed in 80 %, while only 3 % of patients discontinued epcoritamab due to TEAEs that were related to this drug. No grade 5 TEAEs related to epcoritamab occurred. The most common grade ≥ 3 TEAE was neutropenia (51 %), although no neutropenic events led to the discontinuation of epcoritamab. At 69 %, CRS was the most common TEAE; these events were primarily low-grade, and all resolved with tocilizumab and/or corticosteroid treatment. The CRS onset showed predictable timing, with most events occurring after the first full dose.
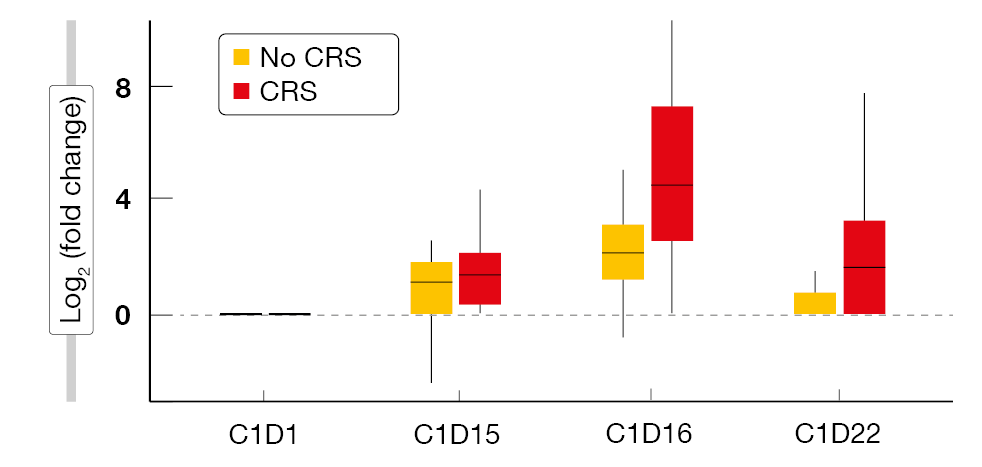
According to a biomarker analysis, the incidence and severity of CRS were reflected by the cytokine levels, as IL-6 peaked on day 16 of cycle 1, i.e., immediately after the first full dose (Figure 2). The analysis revealed similar findings for IL-2 and IFN-γ. One patient developed grade 3 ICANS that resolved after two days. The authors pointed out that these data are the first results of a bispecific antibody in combination with lenalidomide for relapsed/refractory DLBCL and support further exploration of epcoritamab plus lenalidomide in these patients.
Figure 2: Treatment with epcoritamab plus lenalidomide: peak of the IL-6 levels at day 16 of cycle 1
REFERENCES
- Westin et al., Smart Start: Rituximab, lenalidomide, and ibrutinib in patients with newly diagnosed large B-cell lymphoma. J Clin Oncol 2023; 41(4): 745-755
- Westin J et al., Smart Stop: Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone and with combination chemotherapy for the treatment of newly diagnosed diffuse large B-cell lymphoma. ASH 2023, abstract 856
- Tilly H et al., Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 Suppl 5: v116-125
- Al-Sarayfi D et al., R-miniCHOP versus R-CHOP in elderly patients with diffuse large B-cell lymphoma: A propensity matched population-based study. Am J Hematol 2024; 99(2): 216-222
- Olszewski AJ et al., Mosunetuzumab and polatuzumab vedotin demonstrates preliminary efficacy in elderly unfit or frail patients with previously untreated diffuse large B-cell lymphoma. ASH 2023, abstract 855
- Ren Y et al., Polatuzumab vedotin, zanubrutinib and rituximab achieved rapid and deep response in previously untreated frail and elderly diffuse large B-cell lymphoma patients. ASH 2023, abstract 1747
- Bannerji R et al., Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol 2022; 9(5): e327-339
- Kim WS et al., Odronextamab in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL): Results from a prespecified analysis of the pivotal phase II study ELM-2. ASH 2022, abstract 444
- Ayyapan S et al., Final analysis of the phase 2 ELM-2 study: Odronextamab in patients with relapsed/refractory diffuse large B-cell lymphoma. ASH 2023, abstract 436
- Avivi I et al., Subcutaneous epcoritamab plus lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma from EPCORE NHL-5. ASH 2023, abstract 438
© 2024 Springer-Verlag GmbH, Impressum