Waldenström macroglobulinemia: optimizing outcomes in the first and later lines
Effects of transition from ibrutinib to zanubrutinib
The randomized phase III ASPEN study compared the next-generation BTK inhibitor zanubrutinib with ibrutinib in patients with symptomatic, MYD88-mutated Waldenström macroglobulinemia (WM), demonstrating a trend towards better response quality and decreased toxicity in the zanubrutinib arm [1]. Eligible patients who participated in trials of zanubrutinib for the treatment of B-cell malignancies could enroll in the BGB-3111-LTE1 study. This long-term extension was accessible to individuals from comparator arms as well. At ASH 2023, Garcia-Sanz et al. presented results for 47 patients who had transitioned from the ibrutinib arm of the ASPEN trial to zanubrutinib in the LTE1 study [2]. At the time of the analysis, treatment with zanubrutinib had been ongoing for at least 1 year. The outcome assessment included the recurrence of adverse events (AEs) that had emerged on ibrutinib treatment.
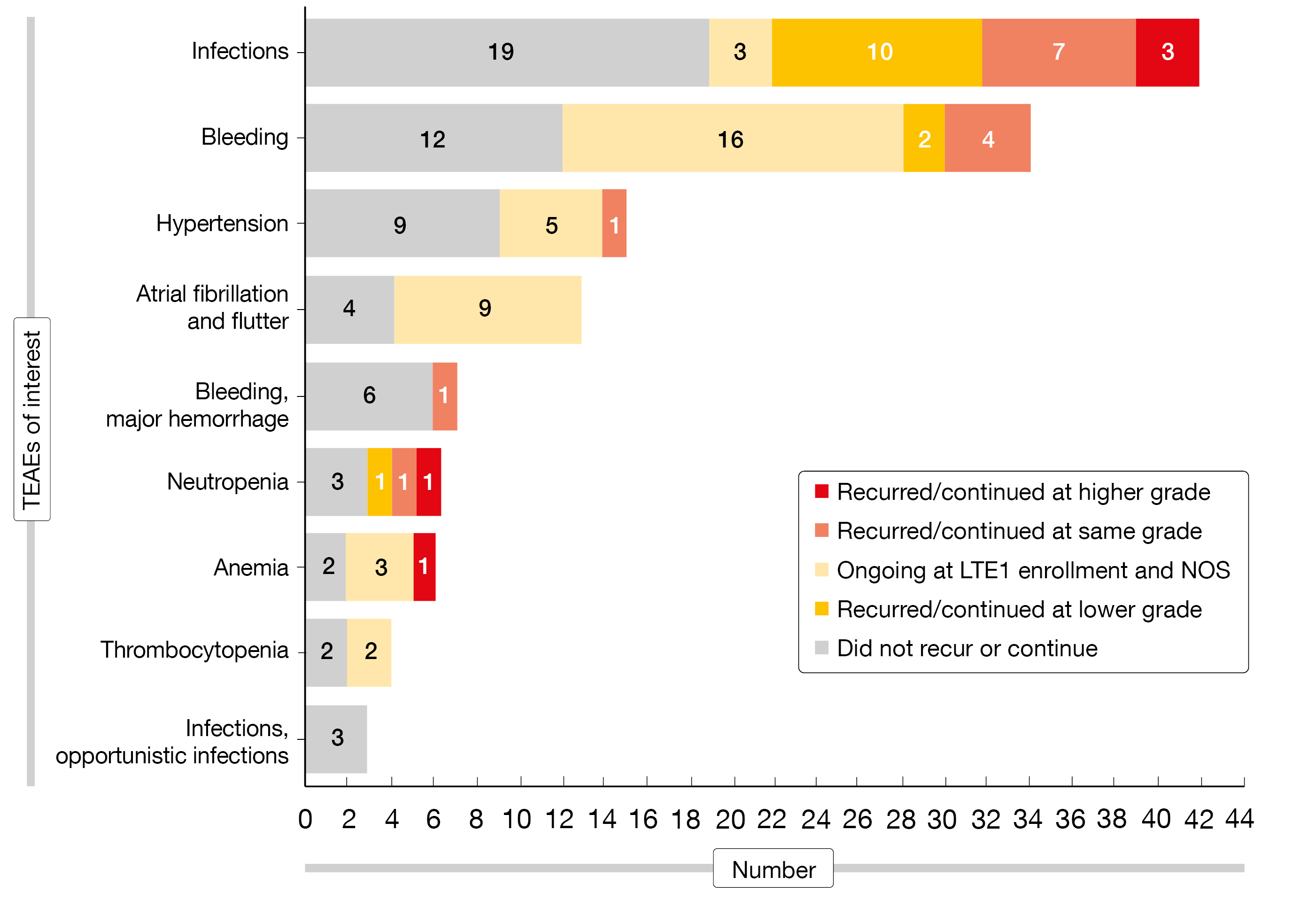
As the safety analysis showed, the majority of ibrutinib-emergent AEs did not recur or continue with zanubrutinib despite increasing patient age (Figure). Worsening of ibrutinib-emergent AEs of interest after the switch to zanubrutinib included three COVID-19 infections and one case each of anemia and neutropenia. Two deaths occurred that were due to COVID-19. Grade ≥ 3 and serious treatment-emergent AEs (TEAEs) were observed in 23 % and 13 %, respectively. The severity of ongoing hypertension did not increase after the switch, and no new or recurrent episodes of hypertension were reported. Likewise, no resolved atrial fibrillation/flutter that had emerged on ibrutinib recurred, and ongoing atrial fibrillation/flutter did not worsen following the transition to zanubrutinib. At 46.8 %, infections were the most common any-grade TEAEs of interest in the LTE1 study; grade ≥ 3 infections occurred in 6.4 %. Hemorrhages were seen in 12.8 % (grade ≥ 3, 2.1 %) and neutropenia in 10.6 % (grade ≥ 3, 4.3 %).
With respect to efficacy, the best overall response in the LTE1 study was unchanged from the last response in ASPEN in 34 patients (72 %) and improved in 10 (21 %). Overall, disease responses were maintained or improved in 96 % of the efficacy-evaluable population. Complete responses (CRs) and very good partial responses (VGPRs) were noted in 4.3 % and 36.2 %, respectively. IgM values were stable or decreased in the majority of evaluable patients. Although this analysis is limited by sample size and the non-randomized design, it suggests that patients who tolerate ibrutinib might switch to zanubrutinib without compromising safety or efficacy and may even experience improvement of their outcomes.
Figure: Recurrence or continuation of ibrutinib-emergent adverse events on zanubrutinib therapy
Frontline bendamustine, rituximab & acalabrutinib
The combination of bendamustine, rituximab and acalabrutinib is being tested in the ongoing BRAWM study as first-line treatment of symptomatic WM with the aim of optimizing CR and VGPR rates. While bendamustine and rituximab are administered for six 28-day cycles, acalabrutinib is taken orally for 1 year. According to interim data reported for 49 patients at ASH 2023, 60 % of participants who reached month 7 achieved CR or VGPR [3]. These rates persisted or improved through month 12 in 80 % and were maintained at month 18 in 89 %. IgM responses occurred already by cycle 3; the results indicated an association of decreases in IgM with increased hemoglobin levels in all study participants. MRD negativity in the peripheral blood was achieved in the total population at cycle 7, and 2.7 to 4.7 log reductions were observed in bone marrow at cycles 7, 12 and month 18. Results in both marrow and blood were sustained throughout the trial.
The most common treatment-related AEs (TRAEs) of the combination included neutropenia, headaches/migraine, fatigue, diarrhea and nausea. Eighteen patients required a total of 31 dose reductions or interruptions during the combination phase. Two discontinued due to TRAEs. Acalabrutinib monotherapy was well tolerated. After a median follow-up of 6 months, no patient has progressed or died. Overall, fixed-duration combination treatment with bendamustine, rituximab and acalabrutinib was shown to be feasible and safe.
Follow-up after ibrutinib and venetoclax cessation
A multicenter, prospective phase II study was initiated to evaluate daily ibrutinib and venetoclax for a maximum of 24 months in treatment-naïve patients with WM. The study was stopped after four ventricular arrhythmia events that included two grade 5 events. Castillo et al. reported follow-up findings for 45 patients after treatment discontinuation to assess ongoing safety and response durability of this combination [4].
Indeed, the combination of ibrutinib and venetoclax was highly effective, with VGPR and partial response rates of 42 % and 53 %, respectively. In patients with CXCR4 wildtype and CXCR4 mutation, VGPR rates were 50 % and 29 %, respectively. The 24-month overall survival and progression-free survival (PFS) rates were 96 % and 76 %, respectively, and the time-to-next-treatment rate at 24 months was 89 %. Additionally, the investigators assessed the outcomes 12 months after the end of treatment. At this time, the analysis showed a PFS rate of 79 %. VGPR attainment appeared to predict longer PFS as the 12-month PFS rate after the end of therapy was 94 % in patients with VGPR but only 69 % in those with < VGPR, although this difference was not significant.
However, the authors noted that they cannot recommend the combination of ibrutinib and venetoclax at the current dose and schedule given that the cause of the unexpected ventricular arrhythmia remains unclear. Nevertheless, the potential for shorter treatment duration or safer combinations offers hope for maintaining efficacy while mitigating adverse effects.
REFERENCES
- Tam C et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020; 136(18): 2038-2050
- Garcia-Sanz R et al., Clinical outcomes in patients with Waldenström macroglobulinemia receiving ibrutinib on the phase 3 ASPEN study ≥ 1 year after transitioning to zanubrutinib. ASH 2023, abstract 3043
- Berinstein NL et al., Indolent lymphoma: high CR and VGPR rate with fixed duration bendamustine, rituximab and acalabrutinib in Waldenströms Macroglobulinemia (BRAWM). ASH 2023, abstract 3037
- Castillo JJ et al., Ibrutinib and venetoclax as primary therapy in symptomatic, treatment-naïve Waldenström macroglobulinemia. ASH 2023, abstract 1661
© 2023 Springer-Verlag GmbH, Impressum