Follicular lymphoma: BTK inhibition and bispecific antibodies
Acalabrutinib in addition to lenalidomide/rituximab
With respect to the treatment of newly diagnosed follicular lymphoma (FL), there is room for improvement as many patients relapse after first-line chemoimmunotherapy. The frontline use of lenalidomide and rituximab (R2) has proven highly active in patients with FL [1, 2]. A single-arm phase II study investigated the addition of the BTK inhibitor acalabrutinib to R2 in patients with untreated FL based on the hypothesis that this combination will increase efficacy due to beneficial effects on the immune microenvironment. Acalabrutinib 100 mg BID was administered orally for 13 cycles; from cycle 2 onwards, the patients received lenalidomide 20 mg on day 1-21 for 12 cycles plus rituximab 375 mg/m2 weekly for four doses followed by monthly administration until month 13. Study participants had FL grade 1–3a, stage III/IV, with high tumor burden. Intermediate and high FLIPI scores were present in 46 % and 25 %, respectively. The best complete response (CR) rate constituted the primary endpoint.
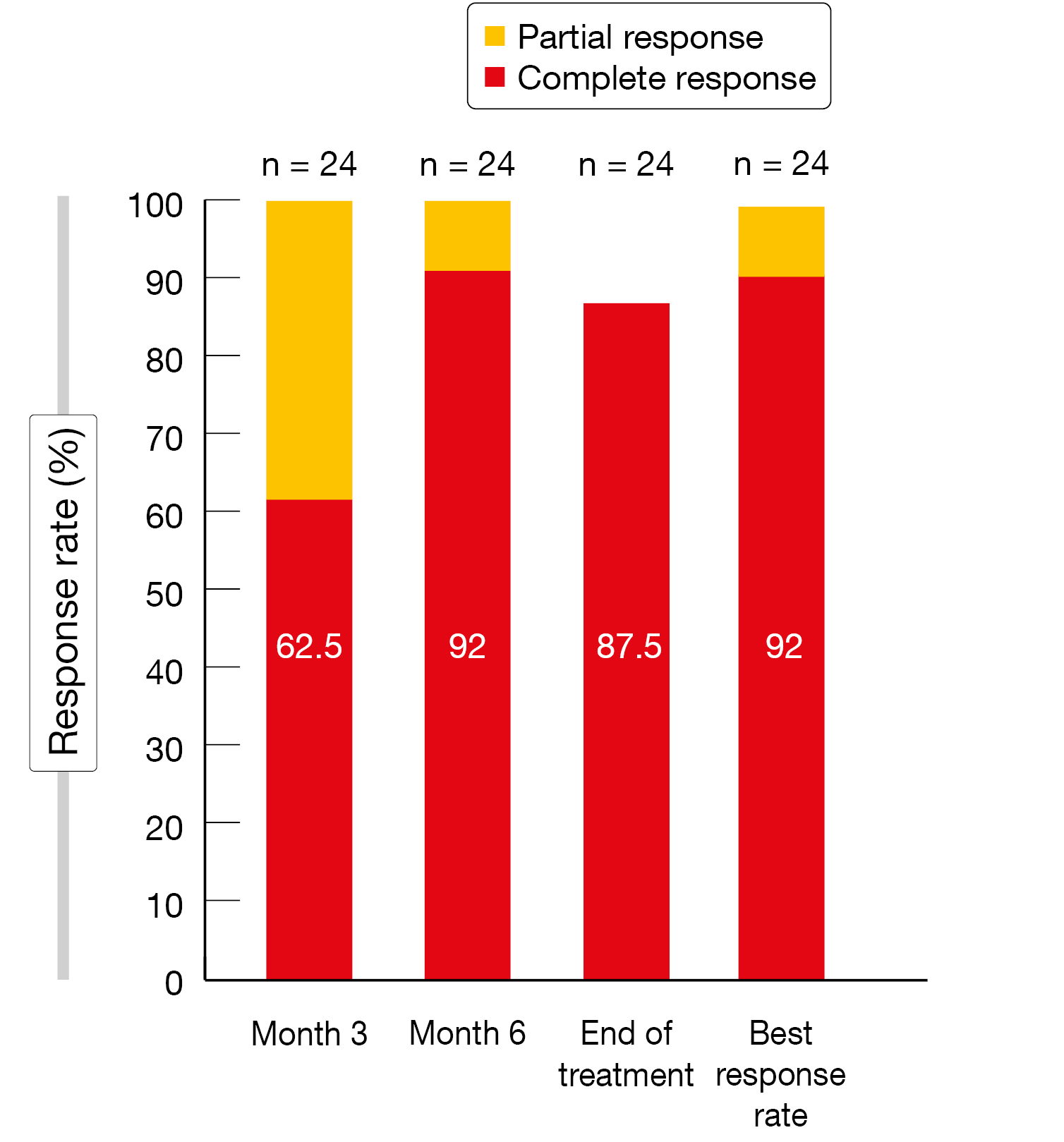
According to the analysis presented by Strati et al. at ASH 2023 for 24 patients, the best CR rate was as high as 92 % (Figure 1) [3]. Already after 3 cycles, 62.5 % of patients achieved CR. At a median follow-up of 29 months, the 2-year overall survival (OS) and progression-free survival (PFS) rates were 92 % and 79 %, respectively. Acalabrutinib plus R2 was shown to be a safe regimen. Treatment-emergent adverse events (TEAEs) mainly included anemia, fatigue, thrombocytopenia, headache and diarrhea. Grade 3/4 AEs were mostly seen for neutropenia, transaminase elevations, infection, anemia and skin rash. Lenalidomide and acalabrutinib dose reduction rates were low at 25 % and 8 %, respectively. The authors concluded that the addition of acalabrutinib to R2 is a safe and effective frontline non-chemotherapy regimen.
Moreover, bulk RNA sequencing with deconvolution performed at baseline and on day 1 of cycles 2 and 6 revealed favorable biological changes in multiple circulating immune cells. Acalabrutinib monotherapy induced significant increases in monocyte-related gene signatures associated with anti-viral activity, monocyte proliferation, monocyte-mediated TNF response, and anti-tumoral activity. The addition of R2 to acalabrutinib significantly increased the frequency of circulating CD4+ T cells and classical monocytes, and decreased the frequency of circulating regulatory natural killer cells. Meanwhile, the study has been amended to include 26 additional FL patients who will be treated with only 6 cycles of acalabrutinib plus R2. Analyses of minimal residual disease (MRD) and progression samples are ongoing.
Figure 1: Response rates for first-line acalabrutinib plus lenalidomide/rituximab
Mosunetuzumab plus lenalidomide
The CD20xCD3 T-cell–engaging bispecific antibody mosunetuzumab is being tested in combination with lenalidomide in an ongoing phase Ib/II study. Previously untreated patients with CD20-positive, grade 1–3a FL are receiving mosunetuzumab subcutaneously Q4W for 12 cycles after step-up in cycle 1. Lenalidomide is being taken orally from cycle 2 to 12. After cycle 12, mosunetuzumab maintenance Q8W for 9 cycles is possible. At ASH 2023, Morschhauser et al. reported preliminary results for 40 patients [4]. Almost 88 % of these had stage III/IV, 42.5 % had FLIPI scores 3–4, and half showed bulky disease.
The combination of mosunetuzumab and lenalidomide demonstrated a manageable safety profile. Injection-site reactions were noted as the most common AE, followed by rash, cytokine release syndrome (CRS) and neutropenia. All of the grade 3/4 AEs reported (55.0 %) were due to neutropenia. No grade 5 events occurred. Dose delays/interruptions of mosunetuzumab and lenalidomide were required in 27.5 % and 42.5 %, respectively. Two AEs (5.0 %) led to treatment discontinuation. Injection-site reactions predominantly emerged in cycles 1 and 2. CRS events occurred in 50 % of patients, were mainly grade 1 and were confined to cycle 1 and early cycle 2. All CRS events resolved, and none necessitated mosunetuzumab discontinuation. Median CRS duration was 2 days.
With an overall response rate (ORR) of 91.9 % and a CR rate of 89.2 %, the combination gave rise to promising anti-lymphoma activity. All responses were still ongoing at the clinical cutoff date. The pharmacokinetic profile of subcutaneous mosunetuzumab following 5/45/45 mg step-up dosing resembled that observed in mosunetuzumab-treated patients with relapsed/refractory FL. In addition, a biomarker analysis was performed that showed early and sustained CD8+ T-cell activation with low PD-1 expression in CD8+ T cells up to cycle 4, although longer follow-up is required.
Taken together, the data support the further development of mosunetuzumab plus lenalidomide as first-line strategy in FL. As the authors noted, this regimen potentially offers outpatient, fixed-duration and chemotherapy-free treatment. The large phase III MorningLyte trial comparing mosunetuzumab plus lenalidomide with chemoimmunotherapy in the first-line setting is being planned.
High-risk disease: EZH2 inhibition
In the setting of high-risk FL, the phase II Epi-RCHOP study was designed to assess the oral EZH2 inhibitor tazemetostat as frontline treatment in addition to chemoimmunotherapy. Patients with FL grade 1–3a, FLIPI scores of 3–5 and high tumor burden were recruited at 23 sites in France and Belgium. They received induction treatment with 6 cycles of R-CHOP and 2 cycles of rituximab monotherapy; tazemetostat 800 mg BID was administered from day 2 of cycle 1. Maintenance consisted of rituximab plus tazemetostat for 3 cycles followed by rituximab alone in cycles 4–12. Thus, tazemetostat was given continuously for one year. The primary endpoint was the complete metabolic response (CMR) rate at the end of induction. EZH2 mutations were found in 24.2 % of the population according to parallel testing of liquid and tissue biopsy. Bone marrow involvement was present in 68.3 %, and 21 % of patients showed blood dissemination.
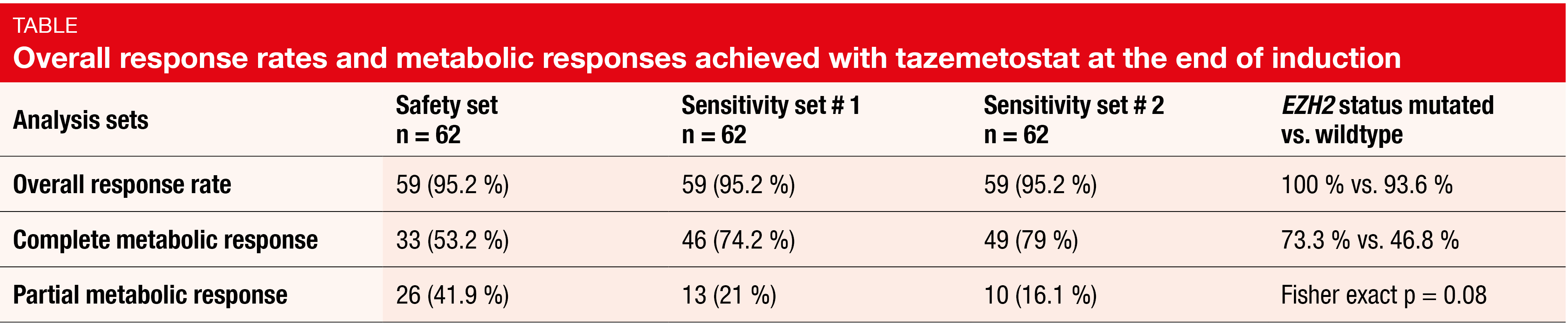
The study did not meet its primary objective of improving the CMR rate to ≥ 80 % [5]. In the safety set (n = 62), the CMR rate was only 53.2 % because many patients lacked bone marrow biopsy at the end of induction and were therefore reclassified as partial metabolic responders. A sensitivity analysis assessing bone marrow involvement by central PET review in patients with partial metabolic responses and another sensitivity analysis considering only lymph nodes and organ responses by PET yielded CMRs of 74.2 % and 79 %, respectively, which again stayed below the 80 % threshold (Table). However, an encouraging finding is the CMR rate of 73.3 % in patients carrying EZH2 mutations (vs. 46.8 % in the group with EZH2 wildtype). As the authors noted, parallel ctDNA/tissue detection of EZH2 mutations allowed for better evaluation of the spatial heterogeneity of the mutational burden. This was deemed a promising tool to tailor targeted therapies and to improve results obtained with frontline chemoimmunotherapy.
At 18 months, 89.3 % of patients were progression-free. To date, no impact of the EZH2 mutation status on PFS has been demonstrated. The hematological safety profile was acceptable for an R-CHOP-based regimen. Dose reductions of vincristine led to improvement of gastrointestinal hypomotility and peripheral neuropathy.
Odronextamab in relapsed/refractory FL
Patients with relapsed/refractory FL often require multiple lines of therapy, with survival decreasing with each line. The CD20xCD3 bispecific antibody odronextamab has shown compelling efficacy and a generally manageable safety profile in heavily pretreated patients with relapsed/refractory FL grade 1–3a in the open-label, multicohort phase II ELM study [6]. Odronextamab 80 mg was administered intravenously on days 1, 8 and 15 of cycles 2–4 after step-up in cycle 1. From cycle 5 onward, odronextamab maintenance consisted of 160 mg Q2W; this was followed by Q4W administration in patients who had achieved CR for ≥ 9 months. The study population was heavily pretreated and highly refractory; they had received ≥ 2 lines of therapy, including an anti-CD20 antibody and an alkylator. Villasboas et al. presented the results of the second prespecified analysis for 128 patients after a median of 19.4 odronextamab treatment cycles [7].
Odrenextamab continued to induce high CR rates. The ORR was 80.5 %, and CR was achieved in 73.4 %. At 12 and 24 months, CR was maintained in 75.8 % and 48.5 %, respectively. Median duration of response and median duration of CR was 22.6 and 23.7 months, respectively. Median OS had not been reached; at 24 months, 69.3 % of patients were alive. In patients with CR, median OS had not been reached at the time of the analysis, while this was 18.4 months in the cohort that obtained partial response (PR). Likewise, median PFS was considerably longer for patients with CR than for those with PR (27.5 vs. 8.0 months). In the total group, median PFS was 20.7 months, with 12-month and 24-month rates of 65.9 % and 45.0 %, respectively.
The safety profile of odronextamab treatment was generally consistent with previous reports. CRS occurred in 56.3 %, neutropenia in 39.8 % and infusion-related reactions in 28.9 %. Median time to CRS onset was 19.7 hours, and CRS events lasted for a median of 2 days. Almost all cases were classified as grade 1 or 2. Treatment-emergent infections of any grade occurred in 79.7 % of patients. COVID-19 infections were reported in 35.9 % and were grade 5 in eight individuals, which is reflective of a study conducted during the pandemic in a population with increased underlying risk for infections. Treatment-related adverse events (TRAEs) led to dose reduction and treatment discontinuation in 9.4 % and 7.8 % of patients, respectively. Patient-reported overall quality-of-life scores were maintained from baseline through week 50. The three randomized phase III trials OLYMPIA-1 (NCT06091254), OLYMPIA-2 (NCT06097364) and OLYMPIA-5 are currently evaluating odronextamab in earlier treatment lines in patients with FL.
First data on epcoritamab
The pivotal EPCORETM NHL-1 study was designed to explore the efficacy and safety of epcoritamab, a subcutaneously administered CD3 x CD20 bispecific antibody, in patients with relapsed/refractory CD20-positive mature B-cell neoplasms after ≥ 2 prior treatment lines including ≥ 1 anti-CD20 antibody, an alkylating agent or lenalidomide. At ASH 2023, first data after a median follow-up of 17.4 months were reported from the EPCORETM NHL-1 FL dose-expansion cohort that included 128 patients [8]. One third of them had previously been treated with ≥ 4 lines, and 42 % had developed progression within 24 months of the initiation of first-line chemoimmunotherapy. Seventy percent were double refractory to both anti-CD20 therapy and an alkylating agent. After step-up in cycle 1, epcoritamab 48 mg was administered until disease progression or unacceptable toxicity.
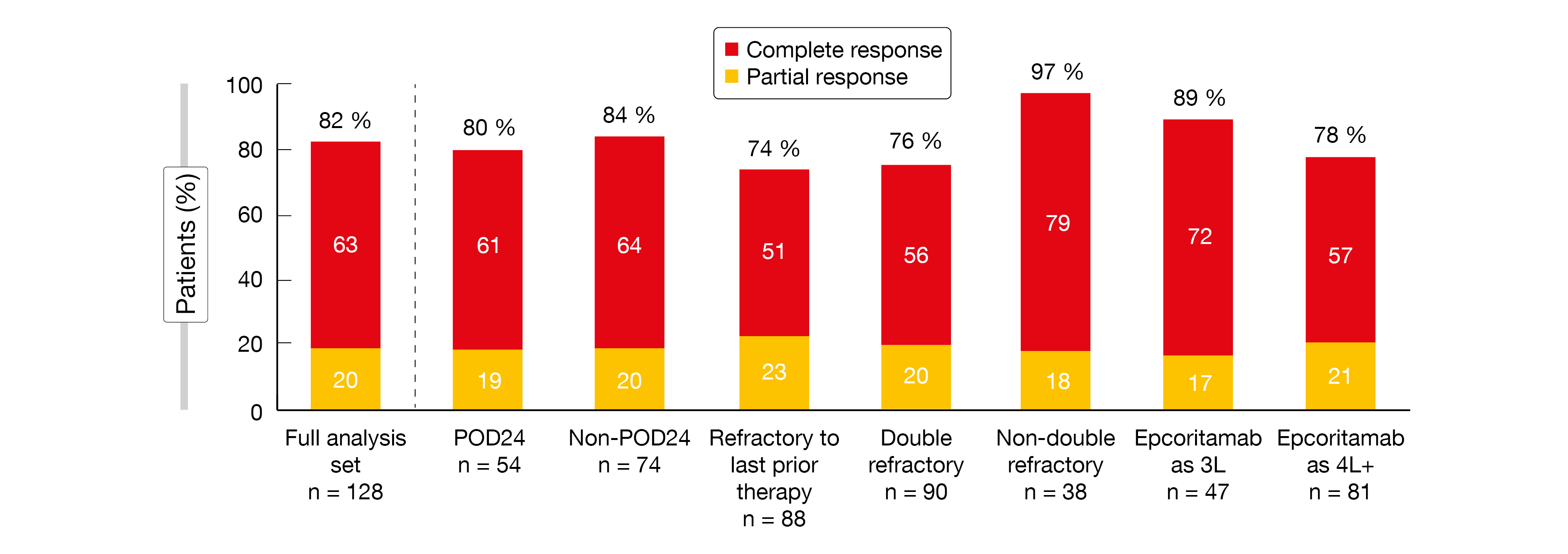
ORRs and CR rates were found to be high regardless of patient subgroup (Figure 2). In the full analysis set, 82 % achieved remission, with 63 % experiencing CR. Responses occurred early on and were deep and durable. Median duration of response had not been reached yet, which also applied to median OS and median time to next treatment. MRD negativity by clonoSEQ® was obtained in 67 % of patients. Median PFS was 15.4 months and had not been reached in the groups achieving CR and MRD negativity.
Common TEAEs such as CRS, injection site reactions and fatigue were mostly low-grade. Grade ≥ 3 TEAEs were reported in 38 % of patients. Neutropenia occurred in 29 %, with grade 3/4 events observed in 26 %. TEAEs led to treatment discontinuation in 19 % of patients; half of these were due to COVID-19. The study contained a C1 optimization cohort (n = 50). Compared to the pivotal cohort, this group received an additional dosing step before the first full dose in cycle 1. Optimization was shown to substantially reduce the risk and severity of CRS.
Figure 2: High overall response rates and complete remission rates with epcoritamab regardless of subgroup; POD24: progression within 24 months of first-line chemoimmunotherapy
Promising findings for pirtobrutinib
Results from the phase I/II BRUIN study highlight the potential of the non-covalent BTK inhibitor pirtobrutinib in the setting of relapsed/refractory FL. BRUIN is assessing pirtobrutinib in patients with various B-cell malignancies. At ASH 2023, Shah et al. presented data from the FL cohort of the study (n = 48) [9]. These patients were pretreated with a median of 3 lines, and 46 % had FLIPI scores of 3–5. More than four nodal sites were involved in 40 %.
Pirtobrutinib demonstrated promising efficacy despite massive pretreatment. Half of all patients responded, with 14.6 % and 35.4 % achieving CR and PR, respectively. Four patients had received prior covalent BTK inhibitor therapy: 3 of these obtained PR, and 1 had stable disease. Median duration of response and median PFS was 5.5 and 5.8 months, respectively. Median OS had not been reached yet. At 24 months, 70.2 % of patients were alive, and 24.2 % were progression-free.
TRAEs included fatigue, nausea, neutropenia, diarrhea and arthralgia, with all of these occurring mainly at grades 1 or 2, except for neutropenia (all grades, 10.4; grade ≥ 3, 8.3 %). Among AEs of interest that are typical of covalent BTK inhibitors, grade ≥ 3 TRAEs were observed for rash only (all grades, 8.3 %; grade ≥ 3, 2.1 %). Infections were reported in 8.3 %, while bruising, hemorrhage and atrial fibrillation/flutter occurred in 2.1 % each. No cases of hypertension were noted. TRAEs necessitated pirtobrutinib discontinuations and dose reductions in 2.1 % and 8.3 %, respectively.
Quality of life in the ROSEWOOD trial
The open-label, randomized phase II ROSEWOOD study evaluated the next-generation BTK inhibitor zanubrutinib in addition to obinutuzumab compared to obinutuzumab monotherapy in patients with heavily pretreated relapsed/refractory FL grade 1–3a. Prior to enrolment, the study population had received ≥ 2 systemic therapies including an anti-CD20 antibody and an alkylating agent. Indeed, the combination has shown superior efficacy over obinutuzumab alone, with a manageable safety profile [10].
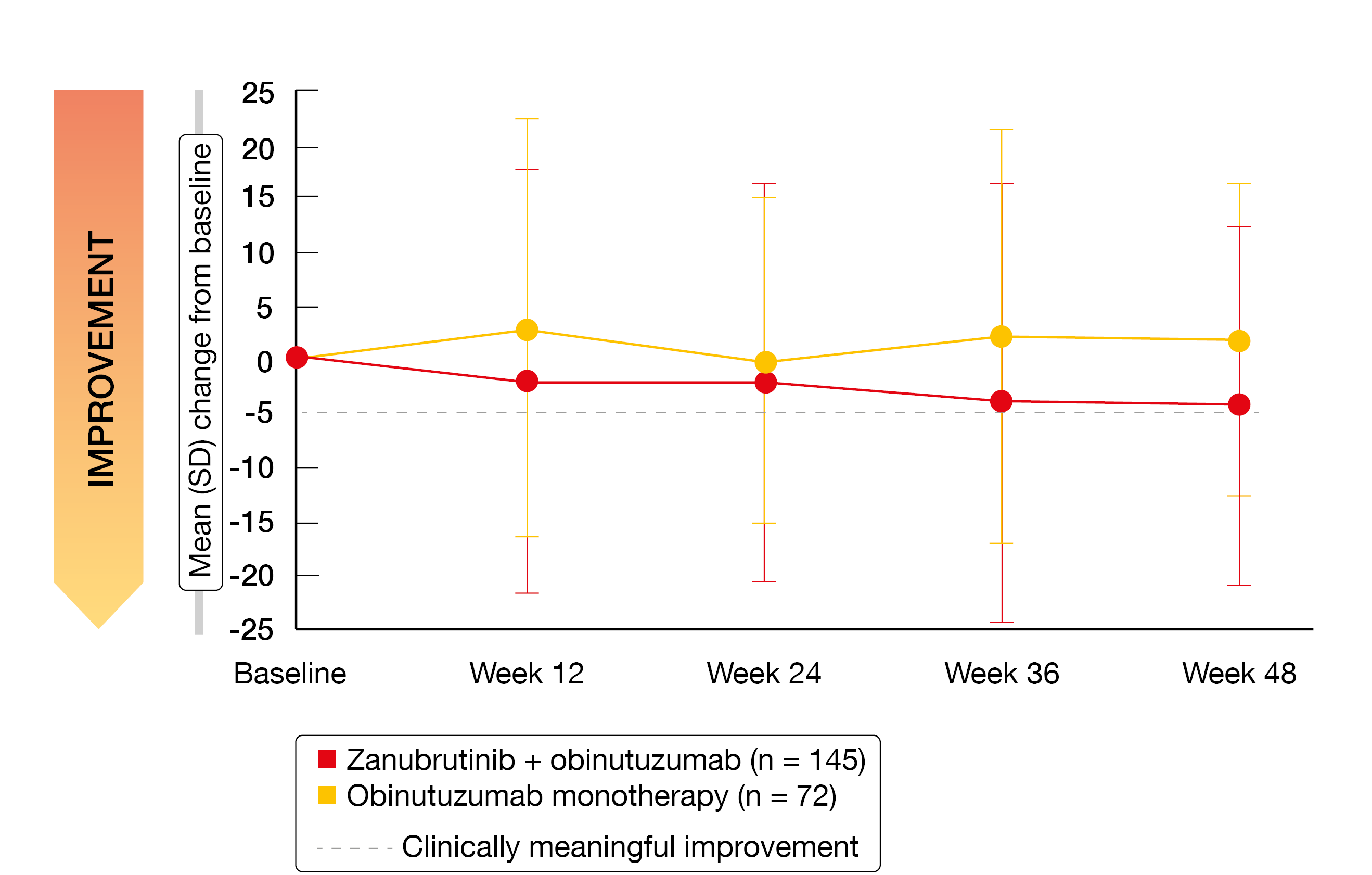
Health-related quality of life, which was assessed using the EORTC QLQ-C30 and the EQ-5D-5L visual analog scale, constituted a secondary endpoint of the study. According to the results of this analysis reported by Trotman et al. at ASH 2023, the patients treated with zanubrutinib plus obinutuzumab (n = 145) derived larger improvements in role functioning and fatigue (Figure 3) through week 48 than those receiving single-agent obinutuzumab (n = 72) [11]. While nausea and vomiting were maintained with the combination, worsening occurred with the monotherapy. No differences resulted regarding physical functioning, pain or diarrhea. Also, the EQ-5D-5L scores did not differ in any meaningful way across the treatment arms.
A mixed model for repeated measures analysis was used to compare the changes in patient-reported outcome endpoints from baseline to the key clinical cycles. This demonstrated clinically meaningful differences between the arms with respect to global health status/quality of life and fatigue at week 12, as well as role functioning, fatigue and pain at week 24. The authors concluded that these findings, along with the primary clinical outcomes, suggest that zanubrutinib plus obinutuzumab is associated with more pronounced clinical and health-related quality of life benefits than obinutuzumab monotherapy in the setting of relapsed/refractory FL.
Figure 3: Improvement of fatigue with zanubrutinib plus obinutuzumab vs. single-agent obinutuzumab in the ROSEWOOD trial
REFERENCES
- Morschhauser F et al., Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 2018; 379(10): 934-947
- Strati P et al., Long-term follow-up of lenalidomide and rituximab as initial treatment of follicular lymphoma. Blood 2021; 137(8): 1124-1129
- Strati P et al., Addition of acalabrutinib to lenalidomide and rituximab induces high complete response rates in patients with previously untreated follicular lymphoma: Results of a phase II study. ASH 2023, abstract 983
- Morschhauser F et al. Preliminary findings of a phase Ib/II trial indicate manageable safety and promising efficacy for mosunetuzumab in combination with lenalidomide in previously untreated follicular lymphoma requiring therapy. ASH 2023, abstract 605
- Ysebaert L et al., Tazemetostat in combination with R-CHOP in patients with high-risk, frontline follicular lymphoma (Epi-RCHOP): A phase II study from the Lysa. ASH 2023, abstract 297
- Kim TM et al., Odronextamab in patients with relapsed/refractory follicular lymphoma grade 1-3a: Results from a prespecified analysis of the pivotal phase II study ELM-2. ASH 2022, abstract 949
- Villasboas JC et al., Results of a second, prespecified analysis of the phase 2 study ELM-2 confirm high rates of durable complete response with odronextamab in patients with relapsed/refractory follicular lymphoma with extended follow-up. ASH 2023, abstract 3041
- Linton KM et al., Epcoritamab SC monotherapy leads to deep and durable responses in patients with relapsed or refractory follicular lymphoma: First data disclosure from the EPCORE NHL-1 follicular lymphoma dose-expansion cohort. ASH 2023, abstract 1655
- Shah NN et al., Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in relapsed/refractory follicular lymphoma: Results from the phase 1/2 BRUIN study. ASH 2023, abstract 3026
- Zinzani PL et al., ROSEWOOD: A phase II randomized study of zanubrutinib plus obinutuzumab versus obinutuzumab monotherapy in patients with relapsed or refractory follicular lymphoma. J Clin Oncol 2023; 41(33): 5107-5117
- Trotman J et al., Health-related quality of life in patients with relapsed/refractory follicular lymphoma treated with zanubrutinib + obinutuzumab versus obinutuzumab monotherapy: The ROSEWOOD trial. ASH 2023, abstract 1674
© 2024 Springer-Verlag GmbH, Impressum