Improving responses in multiple myeloma
PERSEUS: D-VRd vs. VRd
An established treatment approach for transplant-eligible patients with newly diagnosed multiple myeloma (MM) is induction treatment with bortezomib, lenalidomide and dexamethasone (VRd) followed by autologous stem cell transplantation (ASCT), VRd consolidation, and lenalidomide maintenance. The multinational phase III PERSEUS study compared this schedule to an expanded regimen containing the anti-CD38 antibody daratumumab in addition to induction and consolidation with VRd (D-VRd) as well as lenalidomide maintenance (D-R). While 355 patients were treated with D-VRd, 354 received the standard treatment with VRd followed by lenalidomide maintenance. Induction and consolidation consisted of four and two 28-day cycles, respectively. Daratumumab was administered subcutaneously.
Patients enrolled in the experimental arm were able to eventually discontinue daratumumab if they had achieved at least complete remission (CR) and 12 months of sustained minimal residual disease (MRD) negativity after ≥ 24 months of D-R maintenance. Lenalidomide maintenance was continued in this group. Daratumumab treatment could be restarted upon confirmed loss of CR without disease progression or recurrence of MRD. Progression-free survival (PFS) constituted the primary endpoint. Sonneveld et al. presented the primary analysis of the PERSEUS trial at ASH 2023 [1].
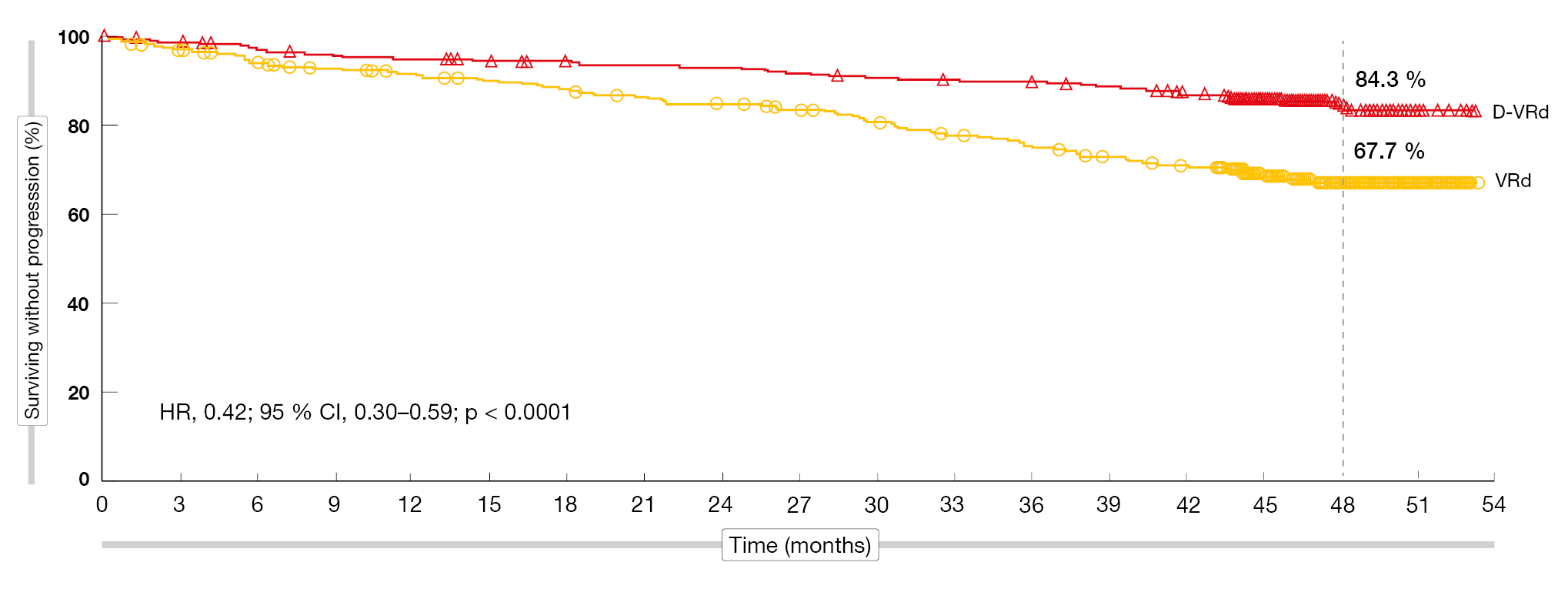
In the D-VRd and VRd groups, 89.5 % and 86.2 % of patients, respectively, completed induction and consolidation, and similar percentages underwent ASCT. Stem cell mobilization and collection were shown to be feasible with D-VRd; the expanded treatment did not impact the patient ability to receive transplant or engraftment. Plerixafor was administered more often in the D-VRd arm, although the number of CD34+ cells collected sufficed for ASCT. Hematopoietic reconstitution resulted in 99.7 % vs. 99.3 %; time to engraftment was 14 days in both arms. In 91.7 % and 86.5 %, respectively, maintenance treatment was initiated. At a median follow-up of 47.5 months, the PFS analysis revealed a 58 % risk reduction in the experimental arm after early separation of the curves, with 48-month PFS rates of 84.3 % vs. 67.7 % (HR, 0.42; p < 0.0001; Figure 1). According to the subgroup analysis, the addition of daratumumab improved PFS across clinically relevant subgroups.
Figure 1: Progression-free survival benefit with D-VRd vs. VRd in transplant-eligible patients
Deep and durable MRD negativity
D-VRd significantly improved the depth of response compared to the VRd regimen. The CR and stringent CR (sCR) rate was significantly higher with D-VRd (87.9 % vs. 70.1 %; OR, 3.13; p < 0.0001). sCRs were present in 69.3 % vs. 44.6 %. A subgroup analysis showed consistent improvement of the > CR rate across subgroups. The combined approach elicited deep and durable MRD negativity. At a sensitivity threshold of 10-5, MRD negativity resulted in 75.2 % with D-VRd vs. 47.5 % with VRd (OR, 3.40; p < 0.0001); at a level of 10-6, this was 65.1 % vs. 32.2 % (OR, 3.97; p < 0.0001). Sustained MRD negativity (10-5) for ≥ 12 months resulted in 64.8 % vs. 29.7 % (OR, 4.42; p < 0.0001). Sixty-four percent of patients receiving maintenance in the D-VRd group were able to discontinue daratumumab after achieving sustained MRD negativity per protocol. MRD negativity rates increased during maintenance, with the absolute difference between D-VRd and VRd widening over time. This was most evident at the threshold of 10-6; here, MRD negativity rates were 34.4 % vs. 16.1 % after consolidation and 65.1 % vs. 32.2 % overall, which translated into differences across the arms of 18 % and 33 %, respectively.
The observed safety profile of the combination was consistent with the known safety profiles for subcutaneous daratumumab and VRd. In both arms, the most common adverse events (AEs) included infections, neutropenia, diarrhea, peripheral sensory neuropathy and thrombocytopenia. As the PERSEUS trial was partly conducted during the pandemic, the infection rates were comparatively high. In their conclusion, the authors noted that these randomized phase III results support D-VRd followed by D-R maintenance as a new standard of care for transplant-eligible patients with newly diagnosed MM.
Isatuximab in addition to KRd
The randomized phase III IsKia EMN24 trial assessed the addition of the anti-CD38 antibody isatuximab to pre-ASCT induction and post-ASCT consolidation with carfilzomib, lenalidomide and dexamethasone (Isa-KRd) in transplant-eligible patients with newly diagnosed MM. Both induction and consolidation involved four 28-day cycles; this was followed by light consolidation consisting of twelve 28-day cycles using reduced-dose KRd and Isa-KRd. In the experimental arm, 151 patients were treated with Isa-KRd, while 151 patients in the control arm received KRd alone. MRD negativity by NGS after post-ASCT consolidation was defined as the primary endpoint. At 42 sites, patients were enrolled between October 2020 and November 2021.
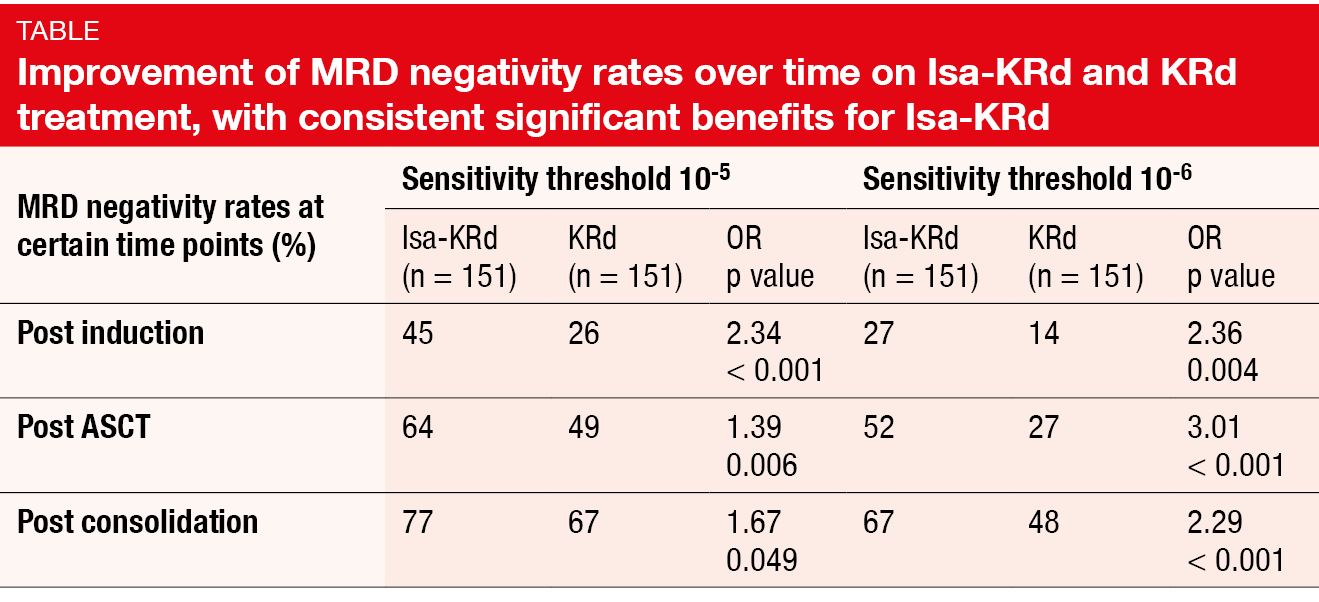
According to the results reported by Gay et al. at ASH 2023 after a median follow-up of 21 months, Isa-KRd, as compared to KRd, significantly increased post-consolidation MRD negativity at sensitivity levels of 10-5 (77 % vs. 67 %; OR, 1.67; p = 0.049) and 10-6 (67 % vs. 48 %; OR, 2.29; p < 0.001) [2]. The MRD negativity rates improved over time in both arms, with significantly higher rates in the experimental arm after induction, transplantation, and consolidation; this applied to both sensitivity levels (Table). Isa-KRd consistently increased MRD negativity at 10-5< and 10-6 in all subgroups including patients with high risk whose results were not inferior to those in the group with standard risk. In the cohort harboring ≥ 2 high-risk cytogenetic abnormalities, i.e. in the patients with very high risk, the post-consolidation MRD negativity rates were 77 % vs. 53 % at the 10-5 level and 77 % vs. 27 % at the 10-6 level.
Isa-KRd proved tolerable, with the toxicity profile being similar to previous observations. Neutropenia occurred as the most common treatment-related adverse event (TRAE) and was more frequent in the experimental arm (41 % vs. 26 %; grade 3/4, 36 % vs. 22 %), although this did not translate into increased infection rates (infections excluding COVID-19, 36 % vs. 32 %; grade 3/4, 15 % vs. 11 %). COVID-19 infections were observed in 26 % vs. 19 %, with most of them being classified as grade 1 or 2. Peripheral neuropathy occurred in 15 % vs. 17 % and was restricted to grade 1 and 2 events. The isatuximab-based treatment did not increase the rate of cardiac disorders (7 % vs. 13 %; grade 3/4, < 1 % vs. 3 %) and thromboembolism (8 % vs. 11 %; grade 3/4, 3 % vs. 6 %). As the authors pointed out, the 10-6 MRD negativity cutoff might be more informative than other response categories in the context of treatment regimens that induce high response rates. With longer follow-up, the IsKia EMN24 trial can offer the opportunity to explore the correlation between the depth of MRD negativity and the survival endpoints PFS and OS.
IFM 2018-04: D-KRd in high-risk patients
Daratumumab was investigated as add-on to KRd induction and consolidation in the single-arm phase II IFM 2018-04 study conducted in high-risk patients with newly diagnosed MM who underwent tandem transplantation. This population showed ≥ 1 of the high-risk cytogenetic features t(4;14), 17p deletion, and t(14;16). Sixty percent of patients presented with ≥ 2 cytogenetic abnormalities. After six D-KRd induction cycles, the first ASCT was performed; this was followed by four D-KRd consolidation cycles and the second ASCT. Finally, the patients received maintenance treatment with daratumumab plus lenalidomide for two years. As eight patients were not able to proceed to tandem transplant due to insufficient stem cell collection, the study protocol was amended to allow for cell collection already after cycle 3 rather than cycle 6. Touzeau et al. reported the findings for 50 patients at ASH 2023 [3].
D-KRd plus double transplant was shown to induce deep responses. CR/sCR was observed in 81 % after the second ASCT. The CR/sCR rate increased in the course of treatment, which also applied to the MRD negativity rate. After the second transplant, 94 % of evaluable patients demonstrated MRD negativity at the 10-6 level. In terms of survival endpoints, the analysis showed that at 30 months, 91 % of patients were alive, with 80 % being progression-free.
Non-hematologic TRAEs of D-KRd mainly included infections (64 %; grade 3/4, 14 %), gastrointestinal disorders (62 %; 10 %), peripheral neuropathy (20 %; 0 %), and skin rash (18 %; 0 %). The most common grade 3/4 TRAEs were hematologic; grade 3/4 neutropenia, thrombocytopenia and anemia occurred in 44 %, 24 % and 22 %, respectively. In four cases, AEs led to the discontinuation of the study treatment. No new safety signals occurred.
Overall, the IFM 2018-04 study confirmed the efficacy and feasibility of D-KRd induction and consolidation with double transplant in high-risk transplant-eligible patients with newly diagnosed MM. The authors emphasized that stem cell collection should be performed 3–4 cycles in the context of anti-CD38 KRd induction to limit the risk of harvest failure, especially in the setting of tandem transplant.
RRMM with t(11;14) translocation: VenDd …
The t(11;14) translocation is the most frequent translocation in MM. In the phase I setting, the combination of venetoclax, daratumumab and dexamethasone (VenDd) has demonstrated preliminary activity in patients with relapsed/refractory MM harboring t(11;14) [4]. Bahlis et al. reported updated findings from the multicenter phase I/II trial at ASH 2023 [5]. In the experimental group, 55 patients were treated with VenDd containing venetoclax at doses of 400 mg or 800 mg OD. They were compared to 26 patients receiving daratumumab, bortezomib and dexamethasone (DVd). All study participants had previously received ≥ 1 prior line of therapy including a proteasome inhibitor or an immunomodulatory agent.
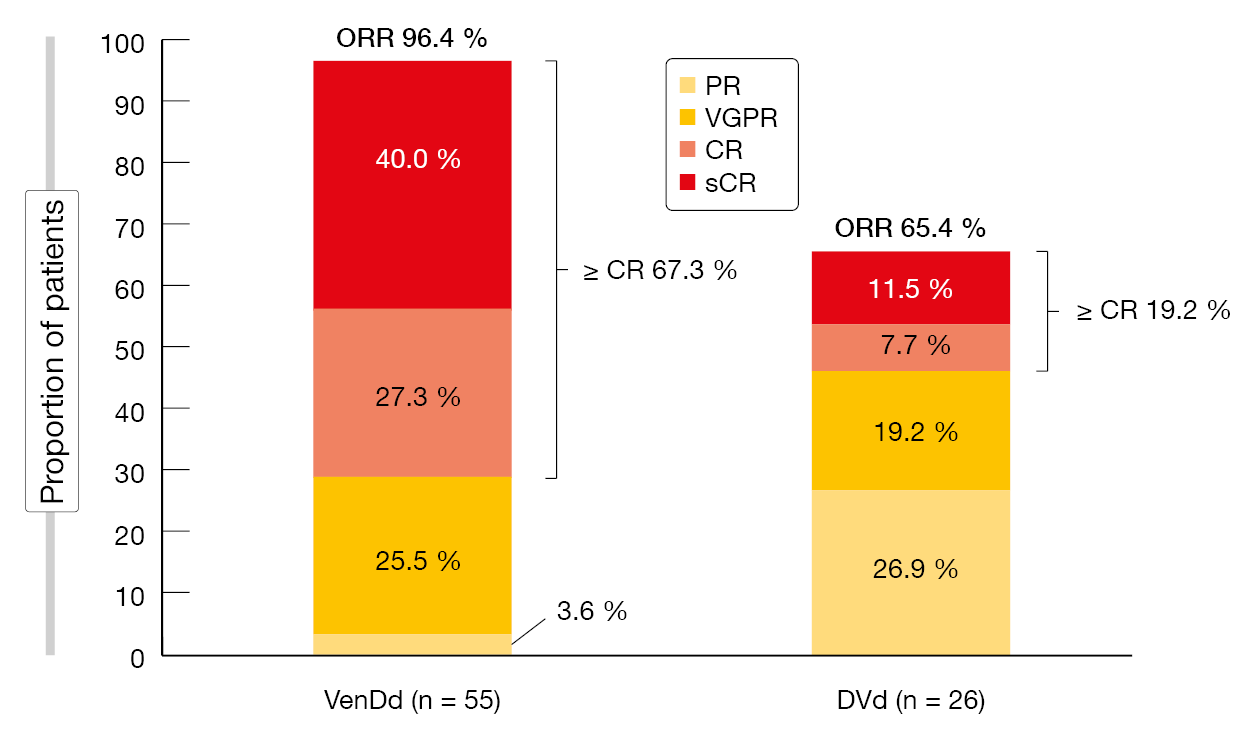
Responses were more pronounced with the venetoclax-based regimen. The overall response rates were 96.4 % vs. 65.4 % for VenDd and DVd, and patients in the experimental arm derived a considerably higher rate of CR/sCR (Figure 2). Similarly, the MRD negativity rates were higher for either dose of VenDd than for DVd at both the MRD < 10-5 cutoff (40 % vs. 8 %) and the 10-6 cutoff (24 % vs. 4 %). VenDd induced higher MRD negativity rates than DVd at both MRD thresholds in key patient subgroups including those with ≥ 2 prior lines of therapy, lenalidomide refractoriness, and high-risk cytogenetics. Moreover, rates of sustained MRD negativity were higher in the experimental arm: Seven of 17 VenDd-treated patients undergoing longitudinal MRD assessments remained MRD-negative for > 6 months, while none of the DVd-treated patients did. Two venetoclax-treated patients achieved MRD negativity for > 12 months.
Median PFS was longer with VenDd than with DVd (46.1 vs. 15.5 months), with 33-month PFS rates of 74.3 % vs. 39.7 %. Within the VenDd arm, analyses according to the MRD results showed a trend towards longer OS in MRD-negative patients (< 10-5) compared to the MRD-positive cohort. The safety analysis demonstrated no new signals. VenDd had a manageable safety profile, with numerically fewer AEs per 100 patient-years compared to DVd (grade 3/4, 134.3 vs. 240.7).
Figure 2: Superiority of VenDd at both doses over DVd regarding response rates
… and sonrotoclax plus dexamethasone
The next-generation Bcl-2 inhibitor sonrotoclax (BGB-11417) has shown more potent and selective Bcl-2 inhibition as well as improved activity against Bcl-2–dependent hematological malignancies than venetoclax in vitro [6]. An ongoing phase I/II study is exploring the combination of sonrotoclax and dexamethasone in patients with t(11;14)-positive MM whose disease has relapsed or is refractory to the most recent treatment line. Participants have failed ≥ 3 prior lines including a proteasome inhibitor, an immunomodulator, and an anti-CD38 antibody. At ASH 2023, preliminary data from the dose-escalation cohorts were presented by Quach et al. [7]. Sonrotoclax was evaluated at doses of 80 mg (n = 3), 160 mg (n = 3), 320 mg (n = 3) and 640 mg (n = 10) in addition to dexamethasone. In the total population of 19 individuals, 68 % have been treated with anti-CD38 antibodies, while all patients have previously received immunomodulatory agents and proteasome inhibitors. The median number of prior treatment lines was 4.
In this heavily pretreated patient group, sonrotoclax plus dexamethasone was well tolerated. The most common treatment-emergent AEs included insomnia, fatigue, nausea, arthralgia, COVID-19, alopecia and diarrhea. Most patients experienced grade 1 or 2 AEs. Diarrhea was manageable with dose interruption. The combination did not give rise to any dose-limiting toxicities at any tested dose level. No significant hematologic toxicity has emerged to date; grade 3 events included one case of decreased lymphocyte count and one case of decreased platelet count. All infections were grade 1 or 2 except for one case of COVID-19.
Investigator assessment of treatment response showed the most promising results for the 640 mg dose after a median treatment duration of 5.5 months, with an overall response rate of 70 % and very good partial response, CR and sCR in 40 % of patients. The longest duration of response at data cutoff was 18.9 months. Sonrotoclax 640 mg has been selected as the recommended phase II dose. At present, recruitment is ongoing for the sonrotoclax plus dexamethasone expansion cohort and for the dose-finding arms investigating sonrotoclax plus dexamethasone and carfilzomib. Further combinations will be assessed later on in this study.
REFERENCES
- Sonneveld P et al., Phase 3 randomized study of daratumumab + bortezomib, lenalidomide, and dexamethasone (VRd) versus VRd alone in patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplantation: primary results of the PERSEUS trial. ASH 2023, LBA-1
- Gay F et al., Results of the phase III randomized IsKia trial: Isatuximab-carfilzomib-lenalidomide-dexamethasone vs. carfilzomib-lenalidomide-dexamethasone and pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. ASH 2023, abstract 4
- Touzeau C et al., Daratumumab carfilzomib lenalidomide and dexamethasone induction and consolidation with tandem transplant in high-risk newly diagnosed myeloma patients: results of the phase 2 study IFM 2018-04. ASH 2023, abstract 207
- Bahlis NJ et al., Phase I study of venetoclax plus daratumumab and dexamethasone, with or without bortezomib, in patients with relapsed or refractory multiple myeloma with and without t(11;14). J Clin Oncol 2021; 39(32): 3602-3612
- Bahlis NJ et al., Venetoclax in combination with daratumumab and dexamethasone elicits deep, durable responses in patients with t(11;14) relapsed/refractory multiple myeloma: Updated analyses of minimal residual disease negativity in phase 1/2 study. ASH 2023, abstract 338
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Quach H et al., Sonrotoclax (BGB-11417) in combination with dexamethasone for the treatment of relapsed/refractory multiple myeloma with t(11;14): Safety, efficacy, and determination of recommended phase 2 dose. ASH 2023, abstract 1011
© 2024 Springer-Verlag GmbH, Impressum
More posts
Exploring chemotherapy-free approaches in the treatment of DLBCL
Exploring chemotherapy-free approaches in the treatment of DLBCL Smart Stop: qu
Updated findings in CLL with a focus on BTK- and Bcl-2–targeted therapies
Updated findings in CLL with a focus on BTK- and Bcl-2–targeted therapies Treat
Follicular lymphoma: BTK inhibition and bispecific antibodies
Follicular lymphoma: BTK inhibition and bispecific antibodies Acalabrutinib in
Improving responses in multiple myeloma
Improving responses in multiple myeloma PERSEUS: D-VRd vs. VRd An established t
Waldenström macroglobulinemia: optimizing outcomes in the first and later lines
Waldenström macroglobulinemia: optimizing outcomes in the first and later lines
Mantle cell lymphoma: emerging treatment regimens and new standards
Mantle cell lymphoma: emerging treatment regimens and new standards SYMPATICO: