Mantle cell lymphoma: emerging treatment regimens and new standards
SYMPATICO: venetoclax plus ibrutinib
Based on promising results of early-phase studies, the multinational, double-blind, placebo-controlled phase III SYMPATICO trial is evaluating the concurrent administration of the BTK inhibitor ibrutinib and the Bcl-2 inhibitor venetoclax in the setting of relapsed/refractory mantle cell lymphoma (MCL) [1, 2]. Patients after 1–5 prior therapies who had received ≥ 1 rituximab/anti-CD20-containing regimen were randomized to either ibrutinib plus venetoclax (n = 134) or ibrutinib plus placebo (n = 133). Venetoclax and placebo were used for 24 months; after that, ibrutinib was continued as a single agent until disease progression or unacceptable toxicity. The primary endpoint is progression-free survival (PFS) by investigator assessment.
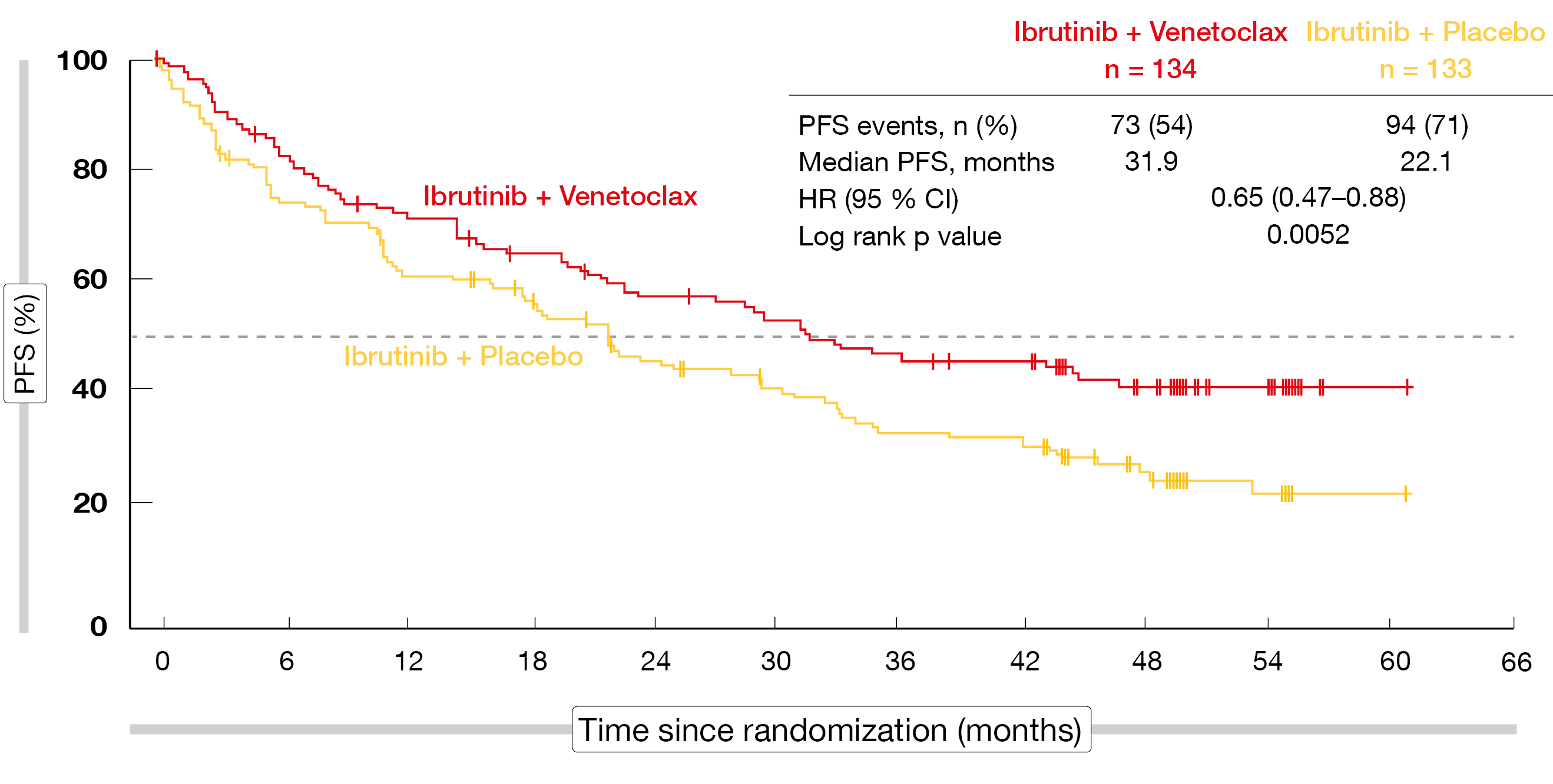
According to the findings reported at ASH 2023 by Wang et al. after a median follow-up of 51.2 months, ibrutinib plus venetoclax improved PFS in a statistically significant manner vs. ibrutinib alone (31.9 vs. 22.1 months; HR, 0.65; p = 0.0052; Figure 1) [3]. The Kaplan-Meier PFS curves separated early on. All sensitivity analyses showed a robust PFS benefit. Moreover, the combination demonstrated significant superiority in terms of time to next treatment (not reached vs. 35.4 months; HR, 0.60; p = 0.0096) and the complete response (CR) rate (54 % vs. 32 %; p = 0.0004). The overall response rates (ORR) did not differ (82 % vs. 74 %; p = 0.1279). Duration of response was significantly longer with ibrutinib plus venetoclax than with ibrutinib plus placebo (42.1 vs. 27.6 months). For overall survival (OS), the analysis revealed numerical improvement (44.9 vs. 38.6 months; HR, 0.85; p = 0.3465).
The safety profile of the combination was consistent with the known adverse events (AEs) of each agent. Any-grade AEs primarily included diarrhea (65 % vs. 34 %), neutropenia (34 % vs. 14 %), nausea (31 % vs. 17 %), fatigue (29 % vs. 27 %), anemia (22 % vs. 12 %) and cough (20 % vs. 27 %). Among grade ≥ 3 AEs, neutropenia was most common (31 % vs. 11 %), followed by pneumonia (13 % vs. 11 %), thrombocytopenia (13 % vs. 8 %) and anemia (10 % vs. 3 %). At 5 %, the rates of grade ≥ 3 atrial fibrillation were identical across the arms. AEs leading to discontinuation of treatment occurred in 31 % vs. 36 %; AE-related dose reductions were necessary in 36 % vs. 22 %.
Ten patients in each arm died due to COVID-19. These fatalities had no meaningful impact on the PFS and OS benefits of the combination. After censoring of COVID-19–related deaths, median PFS was 36.4 vs. 22.1 months (HR, 0.63; p = 0.0042), and median OS was 58.2 vs. 49.4 months (HR, 0.84; p = 0.3257). Overall, the addition of venetoclax to ibrutinib demonstrated a favorable risk-benefit profile in patients with relapsed/refractory MCL. The authors emphasized that this combination represents a new standard of care in this setting.
Figure 1: SYMPATICO trial: significantly improved progression-free survival with ibrutinib plus venetoclax vs. ibrutinib plus placebo
Fixed-duration mosunetuzumab and polatuzumab vedotin
Initial safety and efficacy data were presented from an ongoing phase II expansion cohort of 20 patients with relapsed/refractory MCL who are being treated with the CD20xCD3 T-cell–engaging bispecific antibody mosunetuzumab and the anti-CD79b antibody-drug conjugate polatuzumab vedotin [4]. Mosunetuzumab is administered subcutaneously in 21-day cycles with step-up dosing in cycle 1 for a total of 17 cycles, while polatuzumab vedotin 1.8 mg/kg is administered intravenously on day 1 of cycles 1–6. Prior to each dose in cycle 1, all patients received corticosteroid premedication, and hospitalization was not mandatory. The study participants had previously been treated with ≥ 2 therapies including an anti-CD20 antibody, anthracycline or bendamustine therapy, and BTK inhibition. All patients had received prior BTK inhibitors, while 35 % had undergone CAR T-cell therapy. High-risk features abounded in this population; TP53 aberrations were present in 42 %, blastoid/pleomorphic histology was found in 50 %, and 60 % of patients had Ki-67 index ≥ 50 %.
The study identified no new safety signals in this heavily pretreated cohort. Mosunetuzumab plus polatuzumab vedotin demonstrated a manageable safety profile that was consistent with that of the individual agents in the setting of relapsed/refractory MCL, including disease with high-risk features. Treatment-related AEs (TRAEs) mostly included injection site reactions, all of which were grade 1 or 2, as well as fatigue, cytokine release syndrome (CRS), diarrhea, and dyspnea. CRS events were reported in 45 %, with 40 % and 5 % classified as grade 1 and 2, respectively. Almost all cases occurred in cycle 1, and median CRS duration was 3 days. In 5 % each, corticosteroids or tocilizumab and low-flow oxygen were required to manage CRS. TRAEs led to discontinuation in 10 %. No patient died due to TRAEs.
Regarding efficacy, the combination was shown to induce an ORR of 75 % according to the investigators, with 70 % of patients obtaining CR. The ORR rates were generally consistent across high-risk MCL subgroups including those who had relapsed within 12 months of the first prior therapy, the group that had undergone CAR T-cell therapy before enrolment, and those with TP53 aberrations, among others. CRs were achieved early on and proved durable. After a median follow-up of 15.8 months, 11 of 14 patients with CR remained in remission; median duration of response was estimated at 13.3 months. Median OS and PFS were 17.9 and 15.8 months, respectively. At 9 months, 74.1 % of patients were alive, and 68.8 % were progression-free.
The authors concluded that the fixed-duration outpatient regimen of mosunetuzumab plus polatuzumab vedotin has promising efficacy in a population with relapsed/refractory MCL pretreated with BTK inhibitors. The long-term follow-up will inform the utility of this combination in the treatment landscape.
Pirtobrutinib: update from BRUIN
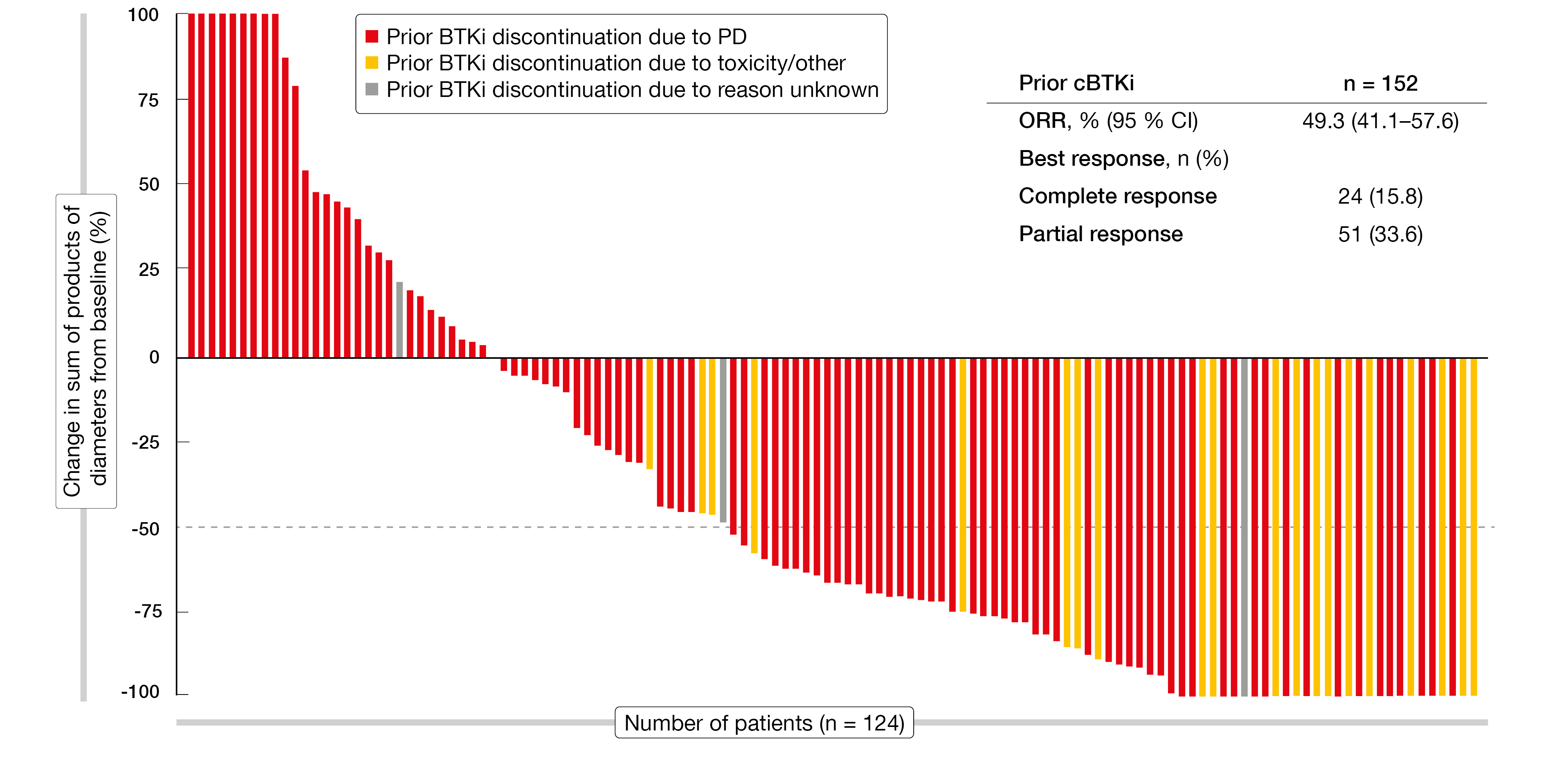
Likewise, the highly selective, non-covalent BTK inhibitor pirtobrutinib has demonstrated efficacy in patients with MCL after covalent BTK inhibition (cBTKi) [5, 6]. Pirtobrutinib was investigated in 166 patients with relapsed/refractory MCL in the phase I/II BRUIN study. Among these, 152 had previously been treated with cBTKi, whereas 14 were cBTKi-naïve. Updated results reported at ASH 2023 by Cohen et al. continued to demonstrate promising efficacy [7]. In the cBTKi-pretreated group, the ORR was 49.3 %, with 15.8 % of patients achieving CR (Figure 2). Median duration of response, median OS and median PFS were 21.6 months, 23.5 months and 5.6 months, respectively. Clinically meaningful ORRs resulted in a variety of MCL subgroups including those with high-risk features such as Ki-67 index ≥ 30 % and bone marrow and gastrointestinal involvement. In the group that had discontinued prior BTK inhibitor therapy due to toxicity, the ORR was very high at 90.5 %, while this was 43.0 % in the cohort of patients who had switched treatment due to progression.
The cBTKi-naïve group showed an ORR of 85.7 %; here, 42.9 % of remissions were classified as CRs. At 24 months, 92.3 % of patients were alive, and 83.9 % were progression-free. Although this cohort is small, these data were deemed encouraging by the investigators. Moreover, pirtobrutinib was well tolerated, with low dose reduction and discontinuation rates due to TRAEs (5 % and 3 %, respectively). Infections, bruising and rash constituted the most common TRAEs of interest (any grade, 15.7 %, 11. 4 %, and 9.0 %, respectively). According to the authors, pirtobrutinib represents a new standard of care for patients with MCL after prior covalent BTK inhibitor treatment. The randomized, global, phase III BRUIN MCL-321 trial is currently comparing pirtobrutinib with covalent BTK inhibitor therapy at the investigator’s discretion in BTK-inhibitor–naïve patients with relapsed MCL (NCT04662255).
Figure 2: Efficacy of pirtobrutinib in patients who received prior covalent BTK inhibitor (cBTKi) therapy
BOVen for patients with TP53-mutant disease
No standard frontline approach has been established to date for patients with MCL harboring TP53 mutations. Survival on treatment with chemoimmunotherapy is poor in this group [8]. Kumar et al. therefore tested the BOVen triplet, i.e., zanubrutinib, obinutuzumab and venetoclax, in a multicenter phase II trial based on the assumption that this regimen will be well tolerated and efficacious in untreated patients with TP53-mutant MCL [9]. Obinutuzumab was administered for a total of 8 cycles, while oral zanubrutinib and venetoclax were continued for a minimum of 24 cycles. Venetoclax was introduced with a standard ramp-up starting on cycle 3, after 2 cycles of obinutuzumab and venetoclax.
At cycle 24, an MRD-driven approach was utilized to limit treatment duration in selected patients. Those who had CR and undetectable minimal residual disease (uMRD) discontinued treatment, while those with < CR and/or detectable MRD continued on zanubrutinib and venetoclax. At two sites, a total of 25 patients were enrolled substantial proportions of whom showed high-risk features such as Ki-67 index ≥ 50 % and high MIPI score. PFS at 2 years was defined as the primary endpoint. As the authors pointed out, this is the first dedicated study for TP53-mutant MCL.
Overall, BOVen proved to be a well-tolerated and safe outpatient regimen. The most frequent AEs comprised diarrhea (any grade, 60 %), COVID-19 infection (48 %), neutropenia (32 %), and infusion-related reactions (24 %). Grade 3 neutropenia occurred in 16 % of patients and resolved with growth factor support. Diarrhea was predominantly grade 1 and manageable. Among serious AEs that were reported in 48 % of patients, COVID-19 infections prevailed (20 %). At cycle 3, only three patients were at high risk for tumor lysis syndrome (TLS) and required initial inpatient venetoclax ramp-up. No clinically significant TLS was observed during ramp-up, although one grade 4 TLS event occurred after the initial administration of obinutuzumab.
uMRD in 80 %
With 72 % of patients being progression-free at 2 years, the primary endpoint of the study was met. This result compared favorably to the 2-year PFS rates observed with standard-of-care regimens in the NORDIC MCL-2 and MCL-3 trials as well as the findings of the SHINE study conducted with bendamustine, rituximab and ibrutinib [8, 10]. At 2 years, 75 % of patients were alive, and 88 % were disease-free. Analyses by baseline factors did not reveal any significant associations between DFS and morphology, age, Ki-67 index, or TP53 mutation/deletion 17. Neither median PFS nor median DFS or OS had been reached at the time of the analysis.
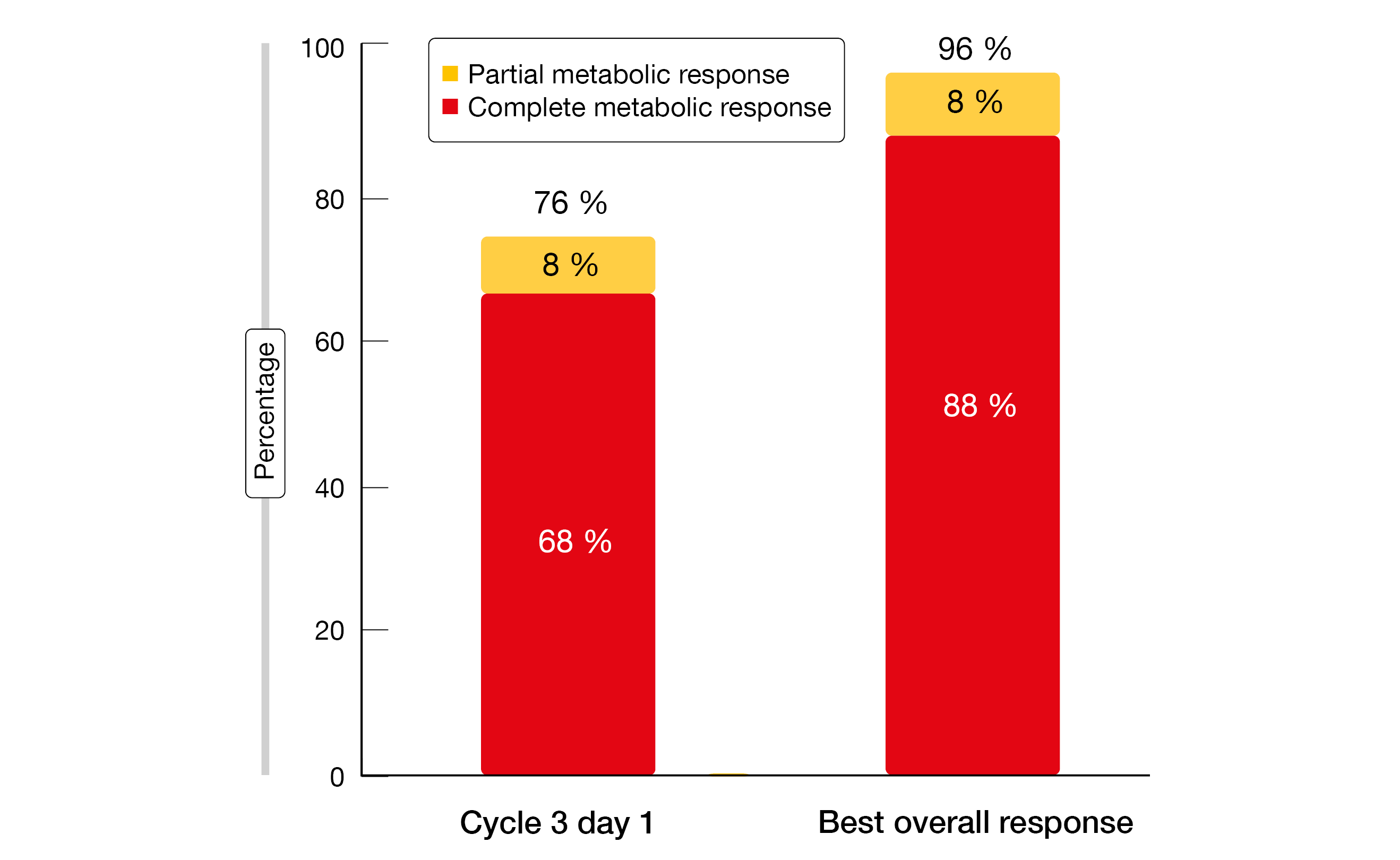
The treatment gave rise to high metabolic response rates already after 2 cycles of zanubrutinib and obinutuzumab (Figure 3). The best ORR on the triplet therapy was 96 %, with CRs achieved in 88 %. All patients had detectable MRD at baseline. By cycle 13, uMRD at the 10-6 level was present in 95 %. Eleven patients completed 24 cycles of therapy, and 100 % of them achieved CR. One patient without MRD results due to the lack of a baseline tumor specimen continued on zanubrutinib and venetoclax. Eight of the 10 remaining patients achieved uMRD and were able to stop treatment. In 6 and 2 of these, uMRD was obtained at sensitivity levels of 10-6 and 10-5, respectively. One patient restarted treatment due to MRD becoming detectable at two time points and another due to clinical relapse. In their summary, the authors noted that BOVen emerges as a promising treatment option for patients with TP53-mutant MCL.
Figure 3: Response rates after two cycles of zanubrutinib and obinutuzumab (left) and best overall response with zanubrutinib, obinutuzumab and venetoclax (right)
Indirect comparison of zanubrutinib and orelabrutinib
A previous indirect comparison of the next-generation BTK inhibitors zanubrutinib and orelabrutinib in Chinese patients with relapsed/refractory MCL has shown that PFS and CR favored zanubrutinib over orelabrutinib [11]. Song et al. reported an updated analysis to indirectly compare the long-term efficacy of the two agents using an unanchored matching-adjusted indirect comparison (MAIC) based on individual patient data from the single-arm BGB-3111-206 and ICP-CL-00102 studies [12]. These two studies included 86 and 106 patients, respectively. Response evaluations were both PET- and CT-based in the zanubrutinib trial and CT-based only in the orelabrutinib trial. After matching, the baseline characteristics were well balanced between the two groups; the effective sample size was 70 for the zanubrutinib cohort.
According to MAIC, zanubrutinib gave rise to statistically significant longer PFS compared to orelabrutinib (not reached vs. 22.0 months; HR, 0.54; p = 0.009). PFS rates were numerically higher for zanubrutinib at 12 months (80.1 % vs. 65.1 % after matching) and 24 months (67.3 % vs. 46.5 %). Although the PET-based assessment is more sensitive than CT in terms of detection of disease progression, PFS according to PET was significantly longer for zanubrutinib than PFS according to CT for orelabrutinib (HR, 0.63; p = 0.044). For OS, a trend was observed in favor of zanubrutinib (HR, 0.68; p = 0.223). The sensitivity analysis results were consistent with the comparative findings.
REFERENCES
- Tam CS et al., Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 2018; 378(13): 1211-1223
- Wang M et al., Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: the safety run-in of the phase 3 SYMPATICO study. J Hematol Oncol 2021; 14(1): 179
- Wang M et al., Ibrutinib combined with venetoclax in patients with relapsed/refractory mantle cell lymphoma: primary analysis results from the randomized phase 3 SYMPATICO study. ASH 2023, abstract LBA-2
- Wang ML et al., Fixed-duration mosun-pola has promising efficacy and a manageable safety profile in patients with prior-BTKi treated relapsed/refractory mantle cell lymphoma: initial results from a phase Ib/II study. ASH 2023, abstract 734
- Hess LM et al., Outcomes among patients with mantle cell lymphoma post-covalent BTK inhibitor therapy in the United States: A real-world electronic medical records study. Adv Hematol 2022; 2022: 8262787
- Mato AR et al., Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet 2021; 397 (10277): 892-901
- Cohen J et al., Pirtobrutinib in relapsed/refractory mantle cell lymphoma patients with prior cBTKi: Updated safety and efficacy including high-risk subgroup analyses from the phase 1/2 BRUIN study. ASH 2023, abstract 981
- Eskelund CW et al., TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017; 130(17): 1903-1910
- Kumar A et al., A multicenter, phase 2 trial with zanubrutinib, obinutuzumab, and venetoclax (BOVen) in patients with treatment-naïve, TP53-mutant mantle cell lymphoma. ASH 2023, abstract 738
- Wang ML et al., Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med 2022; 386(26): 2482-2494
- Song Y et al., Indirect comparisons of efficacy of zanubrutinib versus orelabrutinib in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma or relapsed or refractory mantle cell lymphoma. Invest New Drugs 2023; 41(4): 606-616
- Song Y et al., Indirect comparison of efficacy of zanubrutinib versus orelabrutinib in patients with relapsed or refractory mantle cell lymphoma: An updated analysis with long-term follow-up. ASH 2023, abstract 1682
© 2024 Springer-Verlag GmbH, Impressum