Updated findings in CLL with a focus on BTK- and Bcl-2–targeted therapies
Treatment-naïve disease
Ibrutinib/venetoclax vs. FCR in FLAIR
Personalization of treatment duration of ibrutinib plus venetoclax using measurable residual disease (MRD) was explored in fit patients with previously untreated chronic lymphocytic leukemia (CLL) in the multicenter, randomized, open-label, phase III FLAIR trial. At ASH 2023, Hillmen et al. presented the results for the comparison of ibrutinib plus venetoclax (n = 260) with 6 cycles of fludarabine, cyclophosphamide, and rituximab (FCR; n = 263) [1]. Ibrutinib/venetoclax was administered for 2–6 years depending on the MRD level. Once MRD negativity was achieved, treatment was continued for the same period of time it had taken to obtain undetectable MRD (uMRD). Time to negativity was defined as the first negative result in the peripheral blood that was confirmed by repeated testing after 3 months and then again at 6 months in the peripheral blood and bone marrow. Ibrutinib plus venetoclax was restarted if MRD positivity emerged prior to year 6.
In the arm treated with ibrutinib/venetoclax, as compared to the FCR arm, the rates of complete responses (CRs) and CRs with incomplete count recovery (CRi) were higher at 9 months (59.2 % vs. 49 %) and at any timepoint (92.3 % vs. 71.5 %), as were overall response rates (ORRs) at 9 months (86.5 % vs. 76.4 %) and at any timepoint (95.4 % vs. 83.7 %). uMRD rates in the bone marrow were 61.9% vs. 40.3 %. The majority of patients treated in the experimental arm eventually met the MRD stopping criteria, with the respective percentages rising from 50 % at 27 months to 72.9 % at 51 months. This differed according to the IGHV mutation status, as a higher proportion of patients with unmutated IGHV stopped treatment at 51 months compared to the mutated group (83.0 % vs. 60.4 %).
Significant OS and PFS benefits
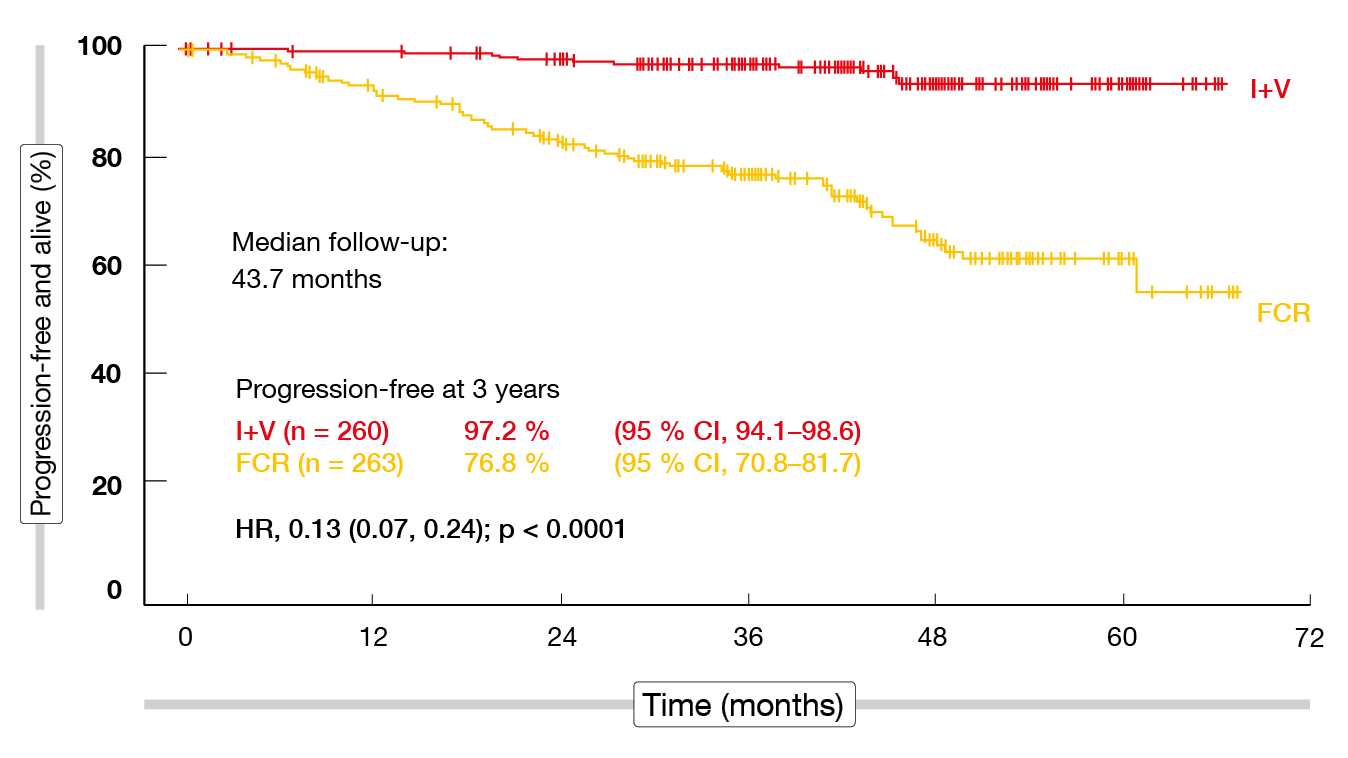
Regarding the primary endpoint, which was progression-free survival (PFS), the analysis yielded an 87 % improvement for ibrutinib/venetoclax vs. FCR after a median follow-up of 43.7 months (Figure 1). PFS rates at three years were 97.2 % vs. 76.8 % (HR, 0.13; p < 0.0001). In addition, ibrutinib/venetoclax gave rise to a significant overall survival (OS) advantage with a 69 % reduction in mortality risk (HR, 0.31; p < 0.005) despite most patients receiving targeted agents as subsequent therapies in the FCR arm. The benefit of the experimental regimen was most obvious in the group with unmutated IGHV; here, the 3-year PFS rates were 98.3 % vs. 70.9 % (HR, 0.07; p < 0.001), and the 3-year OS rates were 99.2 % vs. 93.9 % (HR, 0.23; p = 0.022). At the same time, no differences resulted regarding PFS or OS in the IGHV-mutated subgroups, although the trends favored MRD-guided ibrutinib/venetoclax. Significant advantages of the MRD-guided targeted approach were observed in patients with ATM (11q) deletion, trisomy 12, 13q deletion and normal karyotype. Notably, no patient with 11q deletion has relapsed or died in the study to date.
Ibrutinib/venetoclax was well tolerated, and no unexpected toxicities occurred. While serious AEs of the blood and lymphatic system were more common with FCR (31 % vs. 5.2 %), serious cardiac AEs occurred more frequently with ibrutinib/venetoclax (10.7 % vs. 0.4 %). The incidence of secondary malignancies was twice as high in the FCR arm as in the ibrutinib/venetoclax arm (5.4 % vs. 2.6 % per 100 person-years). Six deaths in the FCR arm and one death in the ibrutinib/venetoclax arm were attributed to treatment. As the authors noted, the exceptional results seen with ibrutinib/venetoclax support the use of MRD to guide duration of therapy to maximize outcomes.
Figure 1: Primary endpoint of the FLAIR study: progression-free survival for ibrutinib/venetoclax vs. FCR
Venetoclax-based combinations: GAIA/CLL13
Updated findings from the phase III GAIA/CLL13 study that assessed venetoclax combinations in the first-line setting were reported at ASH 2023 [2]. This four-arm study investigated rituximab/venetoclax (RV), obinutuzumab/venetoclax (GV), obinutuzumab/ibrutinib/venetoclax (GIV) and chemoimmunotherapy (CIT) that comprised FCR in patients ≤ 65 years or bendamustine-rituximab (BR) in patients > 65 years. RV and GV were administered for a maximum of 12 months. GIV was given in an MRD-guided manner, with patients not achieving uMRD at 15 months receiving ibrutinib maintenance for up to 36 cycles. The uMRD rate at 15 months and PFS constituted the primary objective of the GAIA/CLL13 trial. Overall, 926 treatment-naïve, fit individuals without TP53 aberrations were included. Almost all patients treated with RV and GV stopped treatment after 1 year. In the GIV and CIT groups, early discontinuation rates were 13.4 % and 18.5 %, respectively. Only 6.5 % of patients treated with GIV required ibrutinib maintenance due to MRD persistence.
After a median observation time of 50.7 months, GIV showed superior PFS compared to CIT (HR, 0.30; p < 0.001) and RV (HR, 0.38; p < 0.001). Similarly, GV was significantly more effective than CIT (HR, 0.47; p < 0.001) and RV (HR, 0.57; p = 0.001). At 4 years, the PFS rates for GIV, GV, RV and CIT were 85.5 %, 81.8 %, 70.1 % and 62.0 %, respectively. In patients with unmutated IGHV, PFS was longer with GIV than with GV (HR, 0.58; p = 0.025), RV (HR, 0.40; p < 0.001) and CIT (HR, 0.27; p < 0.001). In contrast, no significant differences were observed between GIV and GV in patients with mutated IGHV. OS did not differ across the four treatment arms; the 4-year OS rates ranged from 93.5 % to 96.2 %.
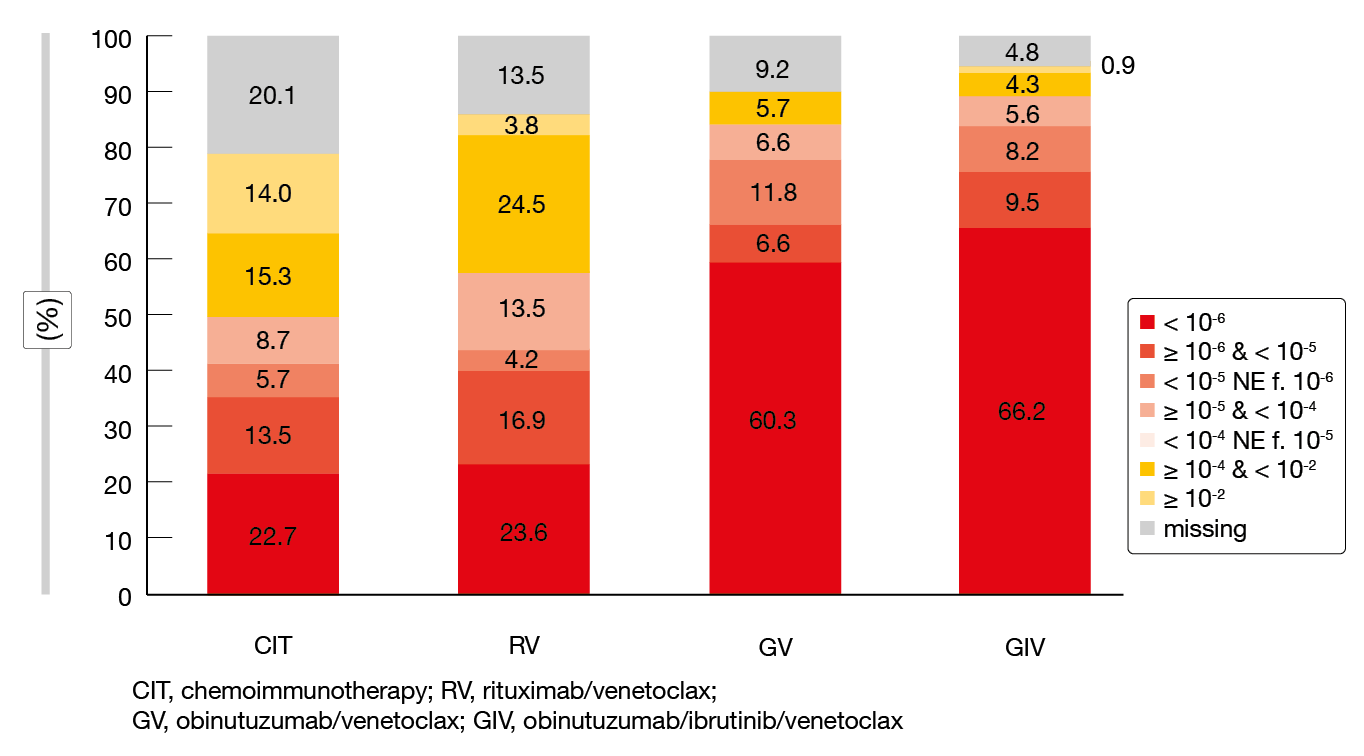
Time to next treatment (TTNT) was significantly prolonged with GIV vs. CIT (HR, 0.17; p < 0.001), GIV vs. RV (HR, 0.27; p < 0.001), GV vs. CIT (HR, 0.34; p < 0.001), and GV vs. RV (HR, 0.54; p = 0.017). The overall rates of patients requiring subsequent therapies were low in the venetoclax-treated arms in spite of the study treatment being limited to one year. Second-line treatments included BTK inhibitors and/or venetoclax in > 90 % of patients. TTNT from the start of second-line therapies was longest in those receiving BTK inhibition plus venetoclax. uMRD at month 15 at the < 10-6 threshold according to NGS was most pronounced with GIV (66.2 %) and GV (60.3 %; Figure 2). For RV and CIT, this was considerably lower at 23.6 % and 22.7 %, respectively. A landmark analysis assessing the correlation between MRD status and PFS in patients treated with GV and GIV showed that patients with uMRD < 10-6 had significantly longer PFS than those achieving uMRD levels ≥ 10-6 and 10-4. Similar correlations were obtained for the RV- and CIT-treated groups. In patients who obtained uMRD 10-6, PFS did not differ according to the depth of response (i. e., CR vs. PR), which suggests that the clinical response status is of limited prognostic significance in the setting of deep MRD responses.
Adverse events (AEs) and grade 3–5 AEs were most frequent in the CIT group. Among the venetoclax regimens, the triple combination gave rise to the highest rate of infections per 1,000 patient-months, which also applied to cardiac AEs and hypertension, although the absolute numbers were relatively low. The authors concluded that NGS-based MRD detection < 10-6 further improves risk stratification and can be used for early identification of patients with very long PFS.
Figure 2: uMRD rates at month 15 of the GAIA/CLL13 trial with various venetoclax-based combinations and chemoimmunotherapy
GLOW: update at 57 months
The randomized phase III GLOW study was initiated to evaluate fixed-duration ibrutinib plus venetoclax in untreated patients aged ≥ 65 years or < 65 years with CIRS > 6 or creatinine clearance < 70 mL/min. While 106 patients were treated with ibrutinib/venetoclax for 12 cycles, the control arm comprising 105 individuals received chlorambucil/obinutuzumab for 6 cycles. Updated clinical outcomes were presented at ASH 2023 after a median follow-up of 57.3 months [3].
Ibrutinib/venetoclax PFS continued to improve PFS compared to chlorambucil/obinutuzumab, reducing the risk of progression or death by 74 % (HR, 0.256; p < 0.0001). The estimated 54-month PFS rates were 66.5 % vs. 19.5 %. Patients treated with ibrutinib/venetoclax fared comparatively better regarding PFS irrespective of the IGHV or MRD status at the end of treatment. At 42 months post treatment, the PFS rates in the ibrutinib/venetoclax arm remained high at 78 % and 70 % for patients with and without uMRD, respectively. In the control arm, this was 44 % vs. 6 %. Within the experimental arm, achieving uMRD at the end of treatment appeared to be more critical for long-term PFS in patients with unmutated IGHV than in those with mutated IGHV. In the unmutated group, the 42-month PFS rates were 78 % and 50 % for patients with and without uMRD, respectively, while these rates did not differ in the mutated IGHV group (91 % and 92 %, respectively).
At the end of treatment with ibrutinib/venetoclax, uMRD was present in 54.7 %. Over time, the rates declined, although approximately two thirds of patients retained uMRD over three years. An analysis of uMRD dynamics according to IGHV status showed that the maximum uMRD rate was approximately 40 % in patients with mutated IGHV and remained stable for three years after treatment completion. In those with unmutated IGHV, on the other hand, the uMRD rate was approximately 60 % at the end of treatment but decreased thereafter, with half of patients losing their remission status. TTNT was significantly longer in the experimental arm, resulting in an 82 % reduction in the risk of requiring second-line therapy (HR, 0.185; p < 0.0001). The 54-month TTNT rates were 87.9 % vs. 54.0 %. Likewise, treatment-free survival was significantly prolonged (54-month rates, 75.6 % vs. 34.5 %; HR, 0.303; p < 0.0001).
The ibrutinib-based treatment continued to improve OS at the prolonged follow-up, reducing the risk of death by 55 % compared to chlorambucil/obinutuzumab (HR, 0.453; p = 0.0038). At 54 months, the OS rates were 84.5 % vs. 63.7 %. Nineteen deaths had occurred in the ibrutinib/venetoclax arm vs. 39 in the chlorambucil/obinutuzumab arm after the 57-month follow-up. Three vs. 13 of these were due to post-treatment infections, and 2 vs. 7 were due to second primary malignancies (SPMs). SPMs occurred in 13.2 % vs. 17.1 % and mainly consisted of non-skin cancers (9.4 % vs. 11.4 %). Hematologic malignancies emerged in 3.8 % vs. 1.0 %.
Long-term results from ELEVATE-TN
Previous analyses from the randomized phase III ELEVATE-TN trial at a median follow-up of 28.3, 46.9 and 58.2 months have established the superiority of the combination of acalabrutinib and obinutuzumab over chlorambucil/obinutuzumab in patients with treatment-naïve CLL [4-6]. The study population was aged ≥ 65 years or > 18 to < 65 years with creatinine clearance 30–69 mL/min and CIRS-G score > 6. ELEVATE-TN was designed as a three-arm study; 179 patients each received either acalabrutinib/obinutuzumab or acalabrutinib monotherapy, while 177 were treated with chlorambucil/obinutuzumab. The primary endpoint was PFS with acalabrutinib/obinutuzumab vs. chlorambucil/obinutuzumab. Sharman et al. presented updated results after a 74.5-month follow-up [7]. At that time, almost half of patients treated with chlorambucil/obinutuzumab had crossed over to single-agent acalabrutinib.
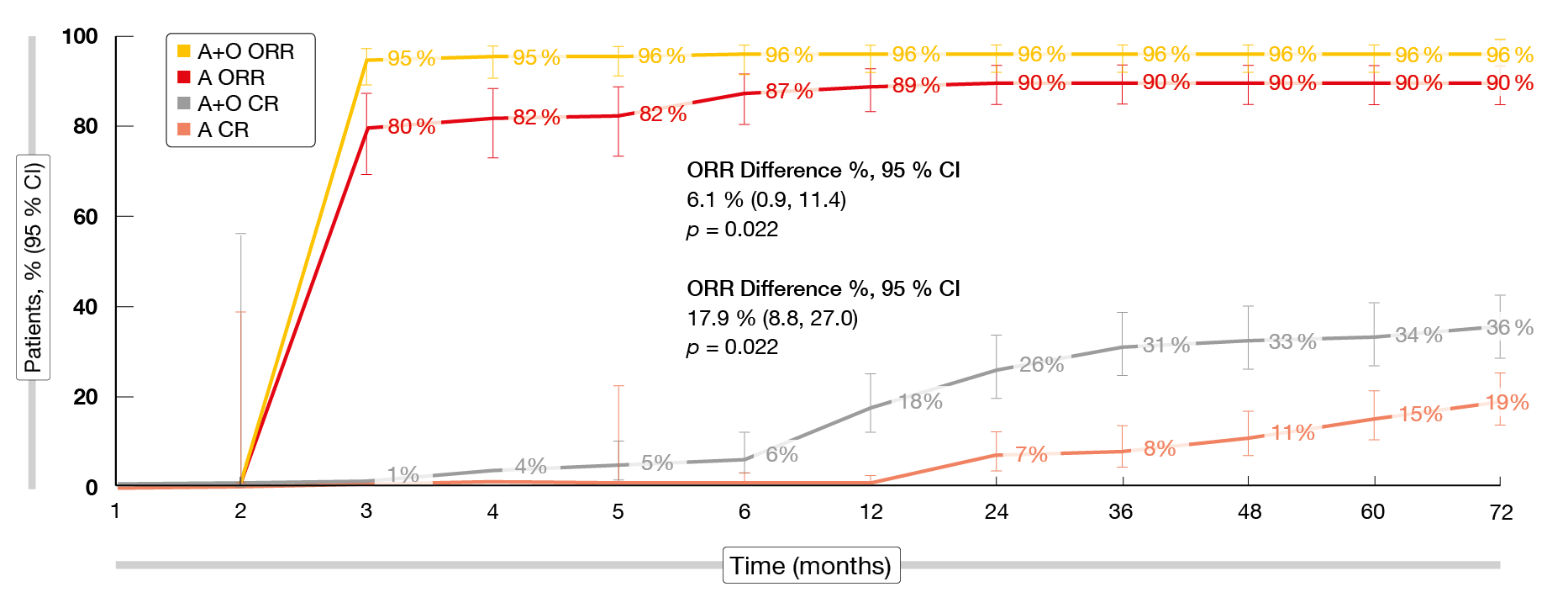
After approximately six years, the efficacy and safety of acalabrutinib/obinutuzumab were maintained. Median PFS was significantly longer for the acalabrutinib-containing arms compared to chlorambucil/obinutuzumab (p < 0.0001 each), with 72-month rates of 78 %, 62 % and 17 % for acalabrutinib/obinutuzumab, acalabrutinib monotherapy and chlorambucil/obinutuzumab, respectively. A post-hoc comparison across the acalabrutinib-containing arms showed that acalabrutinib/obinutuzumab induced significantly longer PFS than acalabrutinib monotherapy (HR, 0.58; p = 0.0229). Obinutuzumab in addition to acalabrutinib resulted in an early ORR benefit versus acalabrutinib alone, although the differences between the acalabrutinib/obinutuzumab and acalabrutinib monotherapy arms narrowed over time (Figure 3). The analysis revealed an absolute ORR difference of 6.1 %. For CR/CRi, this difference was larger at 17.9 %. Both ORR and CR/CRi rates were significantly higher with the combination (p = 0.022 for both comparisons). Moreover, acalabrutinib-treated patients who achieved CR/CRi had significantly longer PFS than those who did not (HR, 0.23; p < 0.0001). Median OS had not been reached in any treatment arm and was significantly longer with acalabrutinib/obinutuzumab vs. chlorambucil/obinutuzumab (HR, 0.62; p = 0.0349). At 72 months, the OS rates were 87 %, 79 % and 80 %, respectively. In the crossover population, median time to second progression had not been reached; the 72-month PFS2 rate was 54 %.
Furthermore, the scientists assessed the impact of molecular risk factors on outcomes. Patients with unmutated IGHV fared significantly worse when treated with chlorambucil/obinutuzumab, while those receiving acalabrutinib/obinutuzumab or acalabrutinib monotherapy were not impacted by the IGHV status. PFS did not differ according to the presence of del(17p) and/or TP53 mutation in patients in the acalabrutinib monotherapy arm; here, the addition of obinutuzumab provided no benefit over single-agent acalabrutinib. The safety of the acalabrutinib-based regimens was consistent with previously reported findings [4-6]. AEs of clinical interest such as cardiac events, bleeding, hypertension and infections were similar for acalabrutinib/obinutuzumab and acalabrutinib monotherapy. After the longer follow-up, the incidence of any-grade atrial fibrillation/flutter and any-grade hypertension remained low in both arms. Neutropenia was more common with acalabrutinib/obinutuzumab than with single-agent acalabrutinib (any grade, 34.3 % vs. 12.8 %), with grade ≥ 3 events noted in 30.9 % vs. 11.7 %.
Figure 3: Overall and complete response rates with acalabrutinib/obinutuzumab vs. acalabrutinib alone over time
Biomarker subgroup analysis of the SEQUOIA data
In the phase III SEQUOIA study, treatment with the next-generation BTK inhibitor zanubrutinib, as compared to BR, has shown superior PFS in treatment-naïve patients with CLL or small lymphocytic lymphoma (SLL) without del(17p) [8]. Xu et al. performed a biomarker analysis to determine the association between molecular features and PFS in SEQUOIA [9].
Zanubrutinib significantly improved PFS compared to BR regardless of cytogenetic abnormalities that included del(11q), del(13q), trisomy 12 and complex karyotype ≥ 3 (p < 0.01). Within the zanubrutinib-treated arm, comparable PFS benefits were observed for patients with or without most of the prognostic biomarkers analyzed. Median PFS in the zanubrutinib arm did not significantly differ between patients with and without IGHV mutation but was significantly improved over the BR arm regardless of the IGHV mutational status. Patients with gene mutations that are associated with poor prognosis such as ATM, BRAF, NOTCH1, and SF3B1 had significantly better PFS with zanubrutinib than with BR. Overall, this study provides further evidence in favor of zanubrutinib as a highly effective BTK inhibitor for frontline treatment of patients with CLL/SLL.
Sonrotoclax plus zanubrutinib
The highly selective, oral, second-generation Bcl-2 inhibitor sonrotoclax (BGB-11417) has shown > 10-fold potency compared with venetoclax in the preclinical setting [10]. In the ongoing phase I/II BGB-11417-101 study, sonrotoclax is being evaluated as monotherapy, in combination with zanubrutinib, and in combination with obinutuzumab with or without zanubrutinib in patients with B-cell malignancies. Tam et al. reported preliminary results for sonrotoclax at two doses in addition to zanubrutinib in a treatment-naïve all-comer population with CLL/SLL [11]. Sonrotoclax 160 mg OD plus zanubrutinib was administered in 51 patients and sonrotoclax 320 mg OD plus zanubrutinib in 56 patients, with 8–12 weeks of zanubrutinib monotherapy preceding sonrotoclax dosing that included a daily or weekly ramp-up schedule. Overall, this was a poor-risk study population. Ten percent were aged ≥ 75 years, and 26 % showed del(17p) and/or TP53 mutation. Approximately one third had increased tumor lysis syndrome (TLS) risk.
Both doses of sonrotoclax in combination with zanubrutinib proved safe and well tolerated. At the time of the analysis, almost all patients remained on treatment. Contusion and neutropenia represented the most common AEs. With the exception of neutropenia and hypertension, AEs were mostly grade 1 and 2. Only one patient required dose reduction due to neutropenia; 17 % received transient treatment with G-CSF. Febrile neutropenia developed in 2 % and resolved without sequelae. Hypertension was not accompanied by any clinical symptoms. No clinical or laboratory TLS occurred with weekly or daily ramp-up. None of the patients developed atrial fibrillation. Diarrhea was mostly grade 1 and did not require any dose reductions. The dose reduction and discontinuation rates of sonrotoclax were low at 5.3 % and 1.1 %, respectively, in the total population.
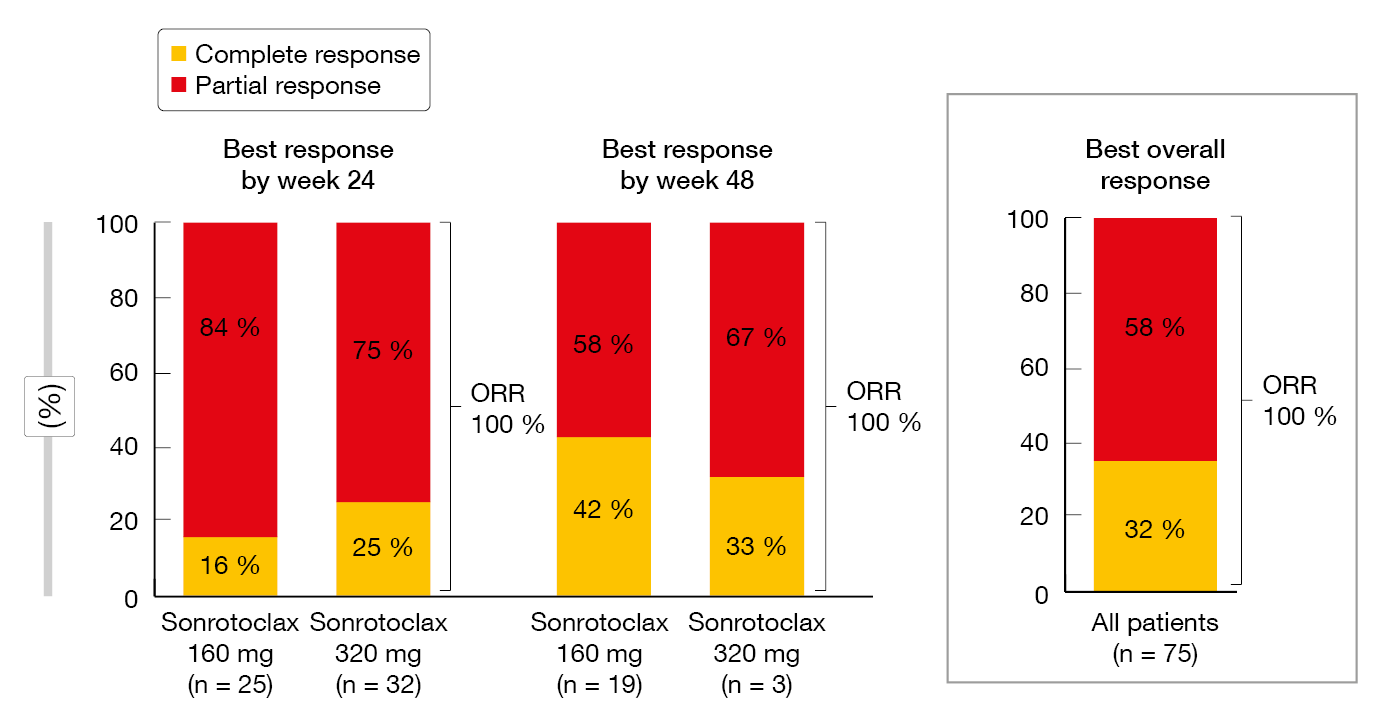
The analysis revealed promising efficacy of sonrotoclax plus zanubrutinib. All of the patients responded by weeks 24 and 48, with depth of response improving over time (Figure 4). At 48 weeks, the CR rates for sonrotoclax 160 mg plus zanubrutinib and sonrotoclax 320 mg plus zanubrutinib were 42 % and 33 %, respectively. High uMRD rates were achieved at both dose levels, although a trend was seen for higher uMRD rates with the 320 mg dose. uMRD4 was achieved in 48 % and 78 % with the 160 mg and 320 mg doses, respectively, at week 24. Again, responses deepened over time. At a median follow-up of 9.7 months, no patient had experienced progression or died at either sonrotoclax dose level. Based on these data, sonrotoclax 320 mg was selected for the phase III study investigating sonrotoclax plus zanubrutinib in patients with treatment-naïve CLL/SLL.
Figure 4: Complete and partial responses observed for sonrotoclax plus zanubrutinib
Relapsed/refractory disease
MRD-guided triple combination: CLL2-BAAG
Patients after 1–4 prior treatment lines were included in the phase II CLL2-BAAG study that evaluated an MRD-guided approach for the triple combination of acalabrutinib, venetoclax and obinutuzumab. This was preceded by optional debulking using bendamustine. Obinutuzumab was started in cycle 1, acalabrutinib in cycle 2 and venetoclax in cycle 3 with initial ramp-up. After 6 cycles, the patients entered the maintenance phase that included a reduced obinutuzumab administration schedule, while the other two agents were continued in an unchanged manner. The duration of maintenance therapy was determined by the MRD status, as patients with uMRD at two consecutive measurements could stop treatment.
At the time of the analysis reported at ASH 2023, all patients were off treatment after a median observation time of 34.4 months [12]. The median treatment duration of 14.7 months observed in this study was considerably shorter than the treatment duration with other options usually prescribed in the relapsed/refractory setting, such as continuous BTK inhibition or venetoclax/rituximab. Few patients had to stop treatment early due to AEs (17.8 %); in most cases, discontinuation was prompted by uMRD that was achieved by 76 % of patients. In those who still had detectable MRD after induction, responses deepened with ongoing treatment. This resulted in a best overall uMRD rate of 93.3 %. Patients who were pretreated with BTK inhibitors and/or venetoclax showed a similarly high uMRD rate of 94.4 %. Median time to uMRD in the peripheral blood was 5.4 months. While most patients developed uMRD within the first months of induction treatment, conversion took longer in some cases, which is why the MRD-guided approach was deemed valuable by the scientists as it ensures the suitable amount of treatment in the individual patient. uMRD translated into durable OS and PFS, with 30-month rates of 100 % and 88.2 %, respectively. Despite the shorter duration of treatment, this compared favorably with other options established in the relapsed/refractory setting, all the more since the study population was enriched for patients harboring TP53 aberrations and those after pretreatment with BTK inhibitors and/or venetoclax.
Moreover, the authors sought to assess whether the addition of circulating tumor DNA (ctDNA)-based MRD measurements to flow cytometry (FCM) contributes to early detection of progression. Overall, 564 paired FCM/ctDNA samples were available. Only five patients have progressed in the study to date. In two of three with CLL-type disease progression, ctDNA was indeed detected before FCM MRD turned positive. In one patient who developed transformation to DLBCL, VDJ ctDNA but not FCM MRD was detected. Taken together, the addition of ctDNA-based analyses to standard FCM MRD assessment appeared to improve the early detection of (molecular) relapses, although the sample sizes were small. ctDNA-based analyses were shown to be particularly beneficial in the setting of more dynamic and advanced disease, as well as in CLL with high-risk genetic features.
ALPINE: 3-year findings
The randomized phase III ALPINE trial was initiated to perform a head-to-head comparison of zanubrutinib 160 mg BID (n = 327) and ibrutinib 420 mg OD (n = 325) in patients with relapsed/refractory CLL/SLL after ≥ 1 systemic treatment. Approximately 23 % in each arm had deletion 17p and/or TP53 mutation, and bulky disease was present in approximately 45 %. As previously reported, zanubrutinib demonstrated significantly improved PFS compared to ibrutinib, with PFS rates of 78.4 % vs. 65.9 % at 24 months (HR, 0.65; p = 0.0024) [13].
The extended findings presented by Brown et al. at ASH 2023 showed sustained PFS benefit of zanubrutinib [14]. After a median follow-up of 39.0 months, the 36-month PFS rates were 64.9 % vs. 54.8 %, which translated into a 32 % risk reduction (HR, 0.68; p = 0.0011). Zanubrutinib outperformed ibrutinib with respect to PFS across all subgroups including patients with del(17p)/TP53 mutation; in this population, the 36-month PFS rates were 58.6 % vs. 41.3 % (HR, 0.52; p = 0.0047). Furthermore, the PFS benefit was consistent across multiple sensitivity analyses. Analyses accounting only for events during active treatment and censoring for new CLL/SLL therapies or for death due to COVID-19 yielded significant risk reductions of 31 % to 34 %. This shows that the PFS advantage observed with zanubrutinib was primarily driven by efficacy and not by tolerability. Responses including CRs deepened over time in both arms, although comparatively higher proportions of patients treated with zanubrutinib achieved CR/CRi at different timepoints. At 48 months, the ORRs were 90.2 % vs. 82.8 % for zanubrutinib vs. ibrutinib, with CR/CRi rates of 10.4 % vs. 7.1 %. The 36-month OS rates were 82.5 % vs. 79.6 % (HR, 0.75).
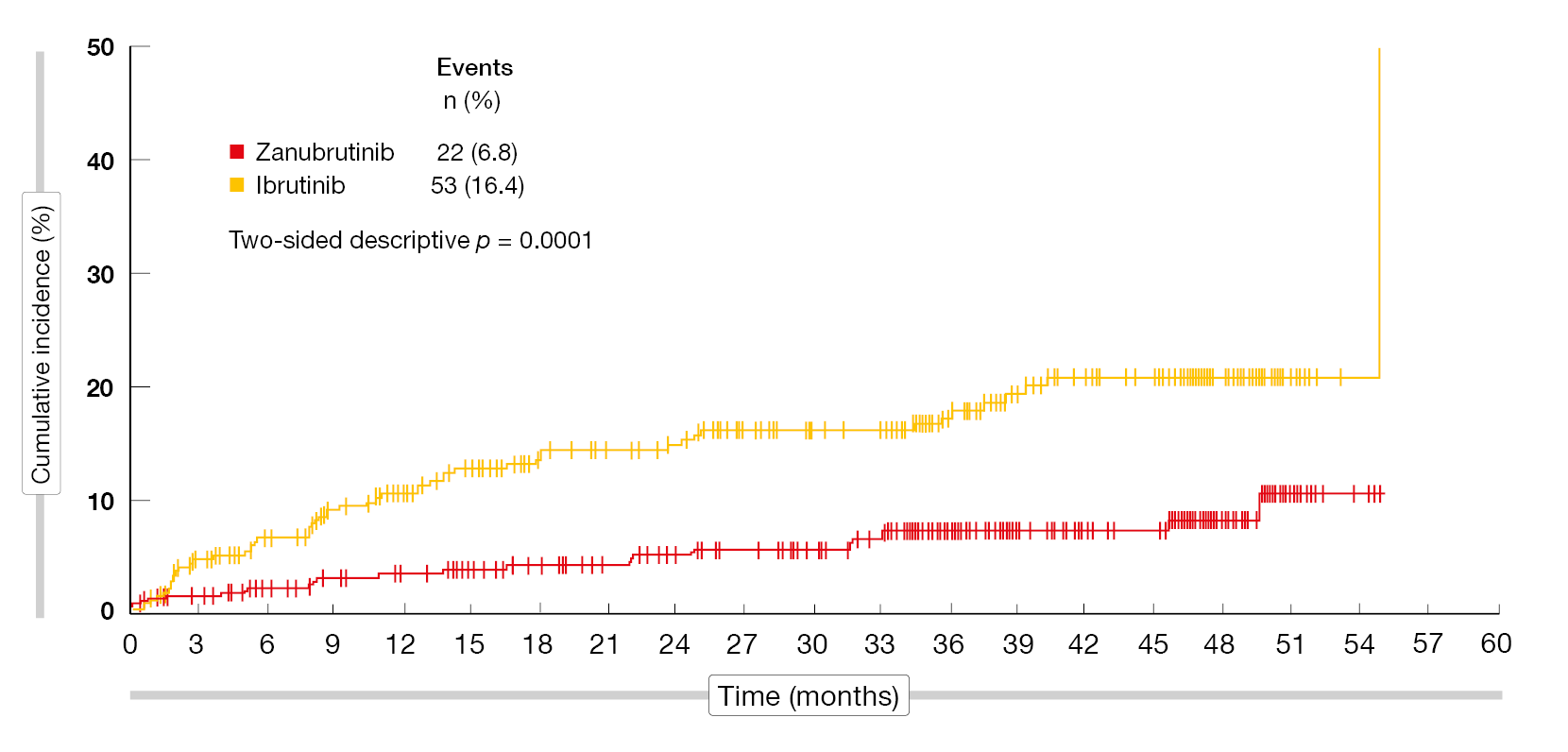
The safety profile of zanubrutinib remained favorable compared to that of ibrutinib. Grade 3–5 AE rates were lower (72.5 % vs. 77.5 %), as were those for serious AEs (50.9 % vs. 59.0 %) and AEs leading to dose reduction (14.5 % vs. 18.2 %), treatment discontinuation (19.8 % vs. 26.2 %), and hospitalization (46.3 % vs. 55.6 %). Zanubrutinib gave rise to lower rates of cardiac AEs (24.7 % vs. 34.6 %) and serious cardiac AEs (3.4 % vs. 9.6 %), as well as cardiac AEs leading to treatment discontinuation (0.9 % vs. 4.6 %) and death (0 % vs. 1.9 %). Atrial fibrillation/flutter occurred significantly less frequently with zanubrutinib than with ibrutinib (6.8 % vs. 16.4 %; p = 0.0001; Figure 5). Despite similar hypertension rates, the absolute change in systolic blood pressure was lower in the experimental arm. The authors concluded that with more than three years of follow-up, these data reconfirm the improved efficacy of zanubrutinib over ibrutinib and a more favorable safety profile in patients with relapsed/refractory CLL/SLL. To date, ALPINE is the only study to demonstrate PFS superiority in a head-to-head comparison of BTK inhibitors.
Figure 5: Significantly fewer atrial fibrillation/flutter events with zanubrutinib vs. ibrutinib
Q-TWiST in ALPINE
Using individual patient data from the ALPINE trial, a quality-adjusted time without symptoms of disease and toxicity (Q-TWiST) analysis was conducted to elucidate the benefits and risks of zanubrutinib vs. ibrutinib. The findings reported at ASH 2023 demonstrated an increase in quality-adjusted survival with zanubrutinib compared to ibrutinib in high-risk patients (n = 73 and 71, respectively) [15]. Zanubrutinib treatment brought about a relative Q-TWiST gain of 9.14 %. This difference was statistically significant across the arms (p < 0.001) and exceeded the relative Q-TWiST gain of 3.03 % observed for acalabrutinib vs. ibrutinib in a similar population included in the ELEVATE-RR study [16].
Time before disease progression with toxicity after randomization was slightly longer with zanubrutinib than with ibrutinib (11.54 vs. 11.38 months). As the authors noted, this could be explained in part by better treatment adherence in the experimental arm of the ALPINE study. Overall, these findings that integrate both the length and quality of survival in addition to efficacy and toxicity provide insights that might help to inform clinical decision-making in the treatment of patients with relapsed/refractory CLL.
Performance of ibrutinib in ALPINE vs. ELEVATE-RR
Both zanubrutinib and acalabrutinib were compared to ibrutinib in the randomized, controlled phase III ALPINE and ELEVATE-RR trials. In ALPINE, zanubrutinib demonstrated superior PFS vs. ibrutinib in an all-comer population, whereas in ELEVATE-RR, acalabrutinib showed non-inferior PFS in patients with del(17p) or del(11q) [13, 17]. In the absence of a head-to-head trial, an unanchored matching-adjusted indirect comparison (MAIC) was performed using a comprehensive list of matching variables to compare the performance of the ibrutinib control arms across ALPINE and ELEVATE-RR. To obtain comparable populations, the high-risk subgroup from ALPINE, i.e., 123 patients with del(17p) or del(11q), was matched against aggregated data from 265 patients in the ibrutinib arm of the ELEVATE-RR trial.
After population adjustment, no statistically significant differences were observed between the ibrutinib arms of the two studies in terms of PFS according to independent review (HR, 0.80; p = 0.3485) and PFS according to investigators (HR, 1.18; p = 0.4827) [18]. Moreover, no statistically significant differences were noted regarding OS (HR, 0.91; p = 0.7539). The results were robust across multiple sensitivity analyses. A scenario matching for additional treatment effect modifiers and prognostic factors also generated results consistent with the main analysis.
Richter transformation: tislelizumab/zanubrutinib
Richter transformation, which describes transformation of CLL from an indolent non-Hodgkin lymphoma into DLBCL or Hodgkin lymphoma, remains a particular unsolved clinical challenge. It occurs in 2–10 % of patients with CLL and involves a median OS of 3–10 months [19-21]. The prospective, multicenter, phase II RT1 trial assessed the combination of continuous zanubrutinib 160 mg BID and the PD-1 inhibitor tislelizumab 200 mg Q3W in patients with Richter transformation. Both induction and consolidation treatment phases comprised six 21-day cycles. Responders were allowed to enter maintenance during which both agents were administered until disease progression. The ORR after induction therapy was defined as the primary endpoint.
At ASH 2023, Al-Sawaf et al. presented the primary analysis of the RT-1 study for 48 patients who constituted the full analysis set [22]. In this group, most had TP53 alteration and/or unmutated IGHV status and/or complex karyotype. The median number of previous CLL-directed therapies was 3. Prior BTK and/or Bcl-2 inhibition had been administered in the majority of patients. One previous treatment line directed against Richter transformation was allowed; this was the case in 20.8 %. Elevated LDH levels were found in 64.6 %. Almost 96 % of patients had DLBCL transformation. The risk according to CLL-IPI was high or very high in 25.6 % and 38.5 %, respectively.
Feasibility of the combined approach
Combined PD-1 checkpoint inhibition and BTK inhibition using tislelizumab and zanubrutinib was shown to be feasible. Infections, which predominantly included COVID-19, as well as hematological toxicities and gastrointestinal AEs were reported most commonly. The majority of AEs were grade 1 or 2. Apart from three cases of fatal sepsis (5.3 %), no grade 5 AEs occurred. No atrial fibrillation was reported. Hypertension worsened in three patients (grade 1–3), and five cases of hematoma occurred. Potentially immune-related events included two cases of hypothyroidism and five cases of liver enzymes increases.
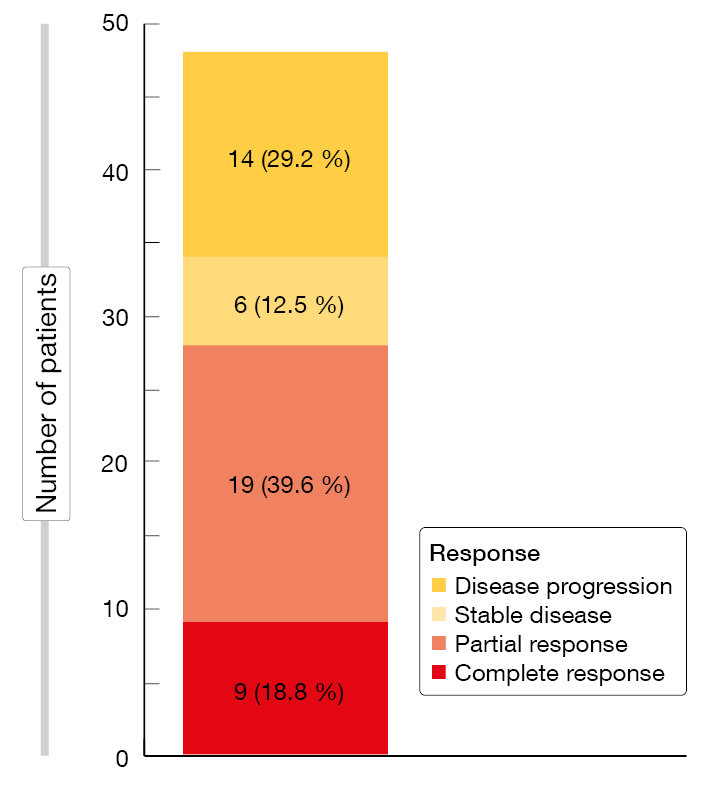
The primary endpoint of the study was met, with an ORR of 58.3 % (Figure 6). Almost 19 % of patients developed CR. At a median observation time of 13.9 months, 19 patients were still on treatment, and most of them had entered the maintenance phase. Median duration of response had not been reached yet, with 70.6 % of patients responding at 6 months. Likewise, median OS had not been reached, while median PFS was 10.0 months. The 12-month OS rate was 74.7 %, and the 12-month PFS rate was 46.9 %.
Subsequent therapies mainly included chemo(immuno)therapy that was administered in 50.0 %. Nineteen percent of patients went on to receive consolidative stem cell transplantation. At present, longer post-transplant follow-up is required to definitely assess long-term outcomes in these patients, although this approach appears feasible. Median TTNT was 12.5 months, with a 12-month TTNT rate of 50.2 %. Furthermore, analyses were conducted to identify predictors of response. Whereas cytogenetic features and patient characteristics were not significantly associated with the ORR, factors associated with PFS included severe constitutional symptoms at presentation, ECOG > 0 and elevated levels of LDH, thymidine kinase and serum β2-microglobulin (> 3.5 mg/L). However, the effect sizes were narrow, and these data are preliminary. A comprehensive workup of all samples is ongoing to enable the identification of patients who might benefit from checkpoint-inhibitor–based treatment. The protocol of the RT1 study has recently been amended to further improve outcomes in an additional cohort treated with tislelizumab plus zanubrutinib and sonrotoclax.
Figure 6: Responses obtained with tislelizumab plus zanubrutinib in the setting of Richter transformation
BRUIN study: pirtobrutinib after covalent BTK inhibition
Treatment with covalent BTK inhibitors is eventually discontinued in the vast majority of patients due to progression or intolerance [23, 24]. Limited treatment options in this setting represent a major unmet medical need in the management of patients with CLL/SLL. Venetoclax-based regimens are frequently used after failure of covalent BTK inhibitors, which leads to an increasing number of patients who are refractory both covalent BTK and Bcl-2 inhibition. In light of poor outcomes, additional treatment options are called for [25].
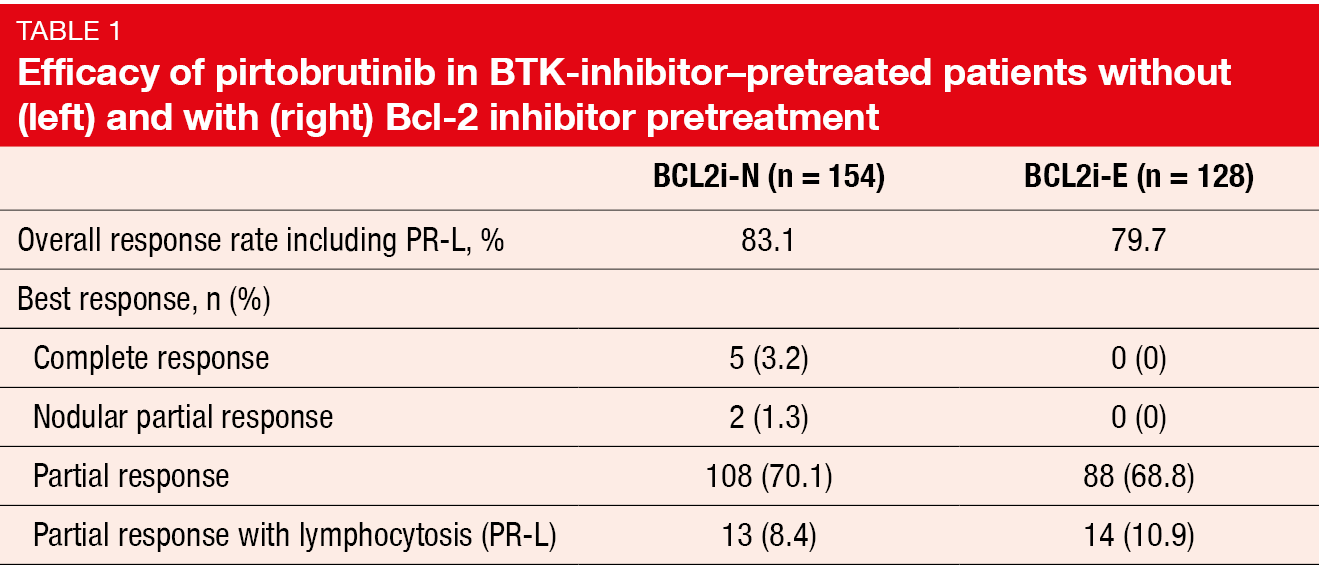
The phase I/II BRUIN study evaluated the highly selective, non-covalent BTK inhibitor pirtobrutinib in 282 patients pretreated with covalent BTK inhibition. Among these, 154 were Bcl-2-inhibitor–naïve (BCL2i-N), while 128 were Bcl-2-inhibitor–exposed (BCL2i-E). The median number of prior lines of systemic therapy was 3 and 5 for the BCL2i-N and BCL2i-E groups, respectively. Also, greater proportions of the Bcl-2-inhibitor–pretreated patients had undergone CAR-T cell therapy (12 % vs. 1 %) and allogeneic stem cell transplantation (5 % vs. 1 %). After the initial dose escalation evaluating pirtobrutinib doses of 25 to 300 mg OD, the expansion phase of the BRUIN study investigated the recommended phase II dose of 200 mg OD. Woyach et al. reported results after a median follow-up of 30 months [26].
According to this update, pirtobrutinib continued to demonstrate clinically meaningful and durable efficacy. The ORR including partial response with lymphocytosis was 81.6 % in all patients, with CRs resulting in 1.8 %. Almost all patients achieved some decrease in lymph node size. A subgroup of 19 individuals after only one therapy prior to pirtobrutinib showed an ORR of 89.5 %. In the BCL2i-N and BCL2i-E cohorts, the ORRs were similar at 83.1 % and 79.7 %, respectively, although CRs were found in the BCL2i-N cohort only (Table 1). Consistent ORR benefits emerged across subgroups in both BCL2i-N and BCL2i-E patients. However, within the BCL2i-N cohort, there was a trend toward a lower ORR for patients with PLCg2 mutation; this also applied to the BCL2i-E group that additionally showed a trend toward a decreased ORR in the presence of IGHV mutation. Responses were increased in the BCL2i-E patients who featured del(17p) and/or TP53 mutation.
While median OS had not been reached in the total group, median PFS was 19.4 months. At 24 months, 73.2 % of patients were alive, and 38.6 % were progression-free. In the BCL2i-N and BCL2i-E cohorts, median PFS was 23.0 and 15.9 months, respectively, while median OS had not been reached in either cohort and the 24-month OS rates were 83.1 % and 60.6 %, respectively. This indicated the availability of effective subsequent options for the BCL2i-N cohort.
Pirtobrutinib treatment was well tolerated, with relatively low overall rates of significant toxicity. Bruising was the most common treatment-related AE (TRAE) of interest (any grade, 19.1 %; grade 3/4, 0 %), followed by infections (any grade, 12.8 %; grade 3/4, 4.3 %). Atrial fibrillation/flutter occurred in 1.4 % (grade 3/4, 0.7 %). Similar safety profiles were seen across the BCL2i-N and BCL2i-E cohorts. Only four and three individuals in the BCL2i-N and BCL2i-E cohorts, respectively, discontinued treatment due to TRAEs. In nine and two patients, respectively, TRAEs necessitated dose reduction. As the authors concluded, these results suggest that continuation of BTK pathway inhibition is an important sequencing approach in the treatment of patients with CLL/SLL.
Pirtobrutinib plus venetoclax ± rituximab
Another pirtobrutinib-based approach explored in the BRUIN study is 2-year, fixed-duration combination treatment with pirtobrutinib/venetoclax (PV, n = 15) and rituximab/pirtobrutinib/venetoclax (PVR; n = 10). While prior covalent BTK inhibition was permitted, prior venetoclax or other Bcl-2 inhibitors were not. Pirtobrutinib and venetoclax were given for 24 cycles. Rituximab was administered in cycles 1–6.
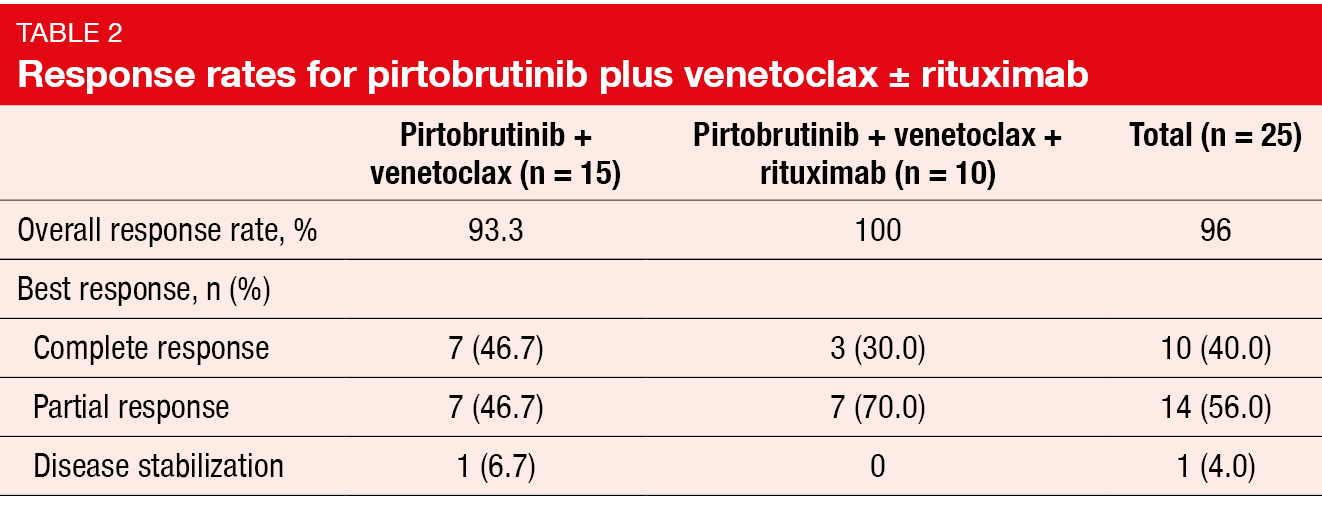
Findings presented at ASH 2023 showed promising efficacy of pirtobrutinib plus venetoclax ± rituximab [27]. The ORRs for PV and PVR were 93.3 % and 100 %, respectively, with CRs of 46.7 % and 30.0 %, respectively (Table 2). Changes in the sum of products of tumor diameters from baseline were similar among BTK-inhibitor–naïve and BTK-inhibitor–pretreated patients. Almost 71 % of patients achieved uMRD at cycle 13, und 87.5 % achieved uMRD at some time during the trial. All but one patient sustained uMRD during subsequent assessments. At 24 months, 79.5 % of the total population were progression-free. Pharmacokinetic analyses demonstrated no apparent drug-drug interactions between pirtobrutinib and venetoclax. Both combination regimens had pharmacokinetic exposures comparable to the monotherapies.
Pirtobrutinib plus venetoclax ± rituximab was well tolerated. No dose-limiting toxicities occurred in either cohort. TRAEs of interest mainly included infections and bruising, and the grade ≥ 3 event rates were very low. Two clinical TLS cases related to venetoclax dose escalation were noted in the PV group, although both patients completed all cycles of combination therapy. The safety profiles were generally similar across the cohorts. At present, the phase III BRUIN CLL-322 trial is comparing PVR with venetoclax/rituximab in previously treated CLL patients (NCT04965493).
Pooled phase Ib/II data on lisaftoclax
The selective Bcl-2 inhibitor lisaftoclax has demonstrated efficacy and tolerability in patients with CLL [28, 29]. Zhou et al. reported updated pooled data for 47 patients with relapsed/refractory CLL who had been treated with lisaftoclax 100 mg, 200 mg, 400 mg, 600 mg and 800 mg OD until disease progression in the phase Ib/II studies APG2575CN001 and APG2575CC101 [30]. A daily ramp-up schedule was used to prevent TLS. Almost half of the population had previously received ≥ 3 treatment lines.
After a follow-up of 14 months, lisaftoclax showed significant efficacy at 400 mg, 600 mg and 800 mg, and potentially at 200 mg. The ORR was 73.3 %, with a CR/CRi rate of 24.4 %. Escalating dose levels were associated with increasing CR/CRi rates; at 400 mg, 600 mg and 800 mg, the rates were 16.7 %, 23.1 % and 33.3 %, respectively. Median OS had not been reached, and the 30-month OS rate was 86.3 %. Median PFS was 18.53 months. Grade 3/4 AEs and serious TRAEs occurred in 68.1 % and 14.9 %, respectively. AEs led to treatment discontinuation in 2.1 %. The analysis identified no significant new or unmanageable safety findings.
REFERENCES
- Hillmen P et al., Ibrutinib plus venetoclax with MRD-directed duration of treatment is superior to FCR and is a new standard of care for previously untreated CLL: Report of the phase III UK NCRI FLAIR study. ASH 2023, abstract 631
- Fürstenau M et al., First-line venetoclax combinations in fit patients with CLL: 4-year follow-up and NGS-based MRD analysis from the phase 3 GAIA/CLL13 trial. ASH 2023, abstract 635
- Moreno C et al., First-line fixed-duration ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab: 57-month follow-up from the GLOW study. ASH 2023, abstract 634
- Sharman JP et al., Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395(10232): 1278-1291
- Sharman JP et al., Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022; 36(4): 1171-1175
- Sharman JP et al., Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Five-year follow-up of ELEVATE-TN. J Clin Oncol 2022; 40 (suppl 16; abstr 7539)
- Sharman JP et al., Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: 6-year follow-up of ELEVATE-TN. ASH 2023, abstract 636
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 2022; 23(8): 1031-1043
- Xu L et al., Broad superiority of zanubrutinib over bendamustine + rituximab across multiple high-risk factors: Biomarker subgroup analysis in the phase 3 SEQUOIA study in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma without del(17p). ASH 2023, abstract 1902
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Tam CS et al., Combination treatment with sonrotoclax (BGB-11417), a second-generation BCL2 inhibitor, and zanubrutinib, a Bruton tyrosine kinase inhibitor, is well tolerated and achieves deep responses in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: Data from on ongoing phase 1/2 study. ASH 2023, abstract 327
- Fürstenau M et al., Long-term remissions with MRD-guided acalabrutinib, venetoclax and obinutuzumab in relapsed/refractory CLL: Follow-up efficacy and ctDNA analysis of the CLL2-BAAG trial. ASH 2023, abstract 203
- Brown JR et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2023; 388(4): 319-332
- Brown JR et al., Extended follow-up of ALPINE randomized phase 3 study confirms sustained superior progression-free survival of zanubrutinib versus ibrutinib for treatment of relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma. ASH 2023, abstract 202
- Levy V et al., Toxicity, progression-free survival, and quality of life of patients treated with zanubrutinib versus ibrutinib: A Q-TWiST analysis from the ALPINE study in relapsed or refractory chronic lymphcytic leukemia. ASH 2023, abstract 1909
- Seymour JF et al., A quality-adjusted survival (Q-TWiST) analysis to assess benefit-risk of acalabrutinib versus idelalisib/bendamustine plus rituximab or ibrutinib among relapsed/refractory chronic lymphocytic leukemia patients. Blood 2021; 138(suppl. 1): 3722-3724
- Byrd JC et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J Clin Oncol 2021; 39(31): 3441-3452
- Shadman M et al., Similar efficacy of ibrutinib arms across ALPINE and ELEVATE-RR in relapsed/refractory chronic lymphocytic leukemia: A matching-adjusted indirect comparison. ASH 2023, abstract 4655
- Al-Sawaf O et al., Richter transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia 2021; 35(1): 169-176
- Davids et al., Richter’s syndrome in patients with chronic lymphocytic leukemia on novel agent therapy. J Clin Oncol 2017; 35(15 suppl): 7505
- Moulin et al., Clinical and biological characteristics and outcomes of Richter transformation : A French multicenter study from the Filo Group. Blood 2019; 134 (Supplement_1): 4112
- Al-Sawaf O et al., Tislelizumab plus zanubrutinib in patients with Richter transformation: primary endpoint analysis of the prospective, multi-center, phase II RT1 trial of the German CLL Study Group. ASH 2023, abstract 204
- Woyach et al., BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol 2017; 35(13): 1437-1443
- Barr PM et al., Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv 2022; 6(11): 3440-3450
- Mato et al. Outcomes for patients with chronic lymphocytic leukemia (CLL) previously treated with both a covalent BTK and BCL2 inhibitor in the United States: A real-world database study. Clin Lymphoma Myeloma Leuk 2023; 23(1): 57-67
- Woyach JA et al., Pirtobrutinib in post-cBTKi CLL/SLL: ~ 30 months follow-up and subgroup analysis with/without prior BCL2i from the phase 1/2 BRUIN study. ASH 2023, abstract 325
- Roeker LE et al., Fixed-duration pirtobrutinib combined with venetoclax ± rituximab in relapsed/refractory chronic lymphocytic leukemia: Updated results, including MRD data, from the BRUIN phase 1b study. ASH 2023, abstract 3269
- Davids MS et al., Lisaftoclax (APG-2575) safety and activity as monotherapy or combined with acalabrutinib or rituximab in patients with treatment-naïve, relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: initial data from a phase 2 global study. Blood 2022; 140(suppl 1): 2326-2328
- Ailawadhi S et al., Novel BCL-2 inhibitor lisaftoclax in relapsed or refractory chronic lymphocytic leukemia and other hematologic malignancies: First-in-human open-label trial. Clin Cancer Res 2023; 29(13): 2385-2393
- Zhou K et al., Updated efficacy and safety results of lisaftoclax (APG-2575) in patients with heavily pretreated chronic lymphocytic leukemia: Pooled analyses of two clinical trials. ASH 2023, abstract 1900
© 2024 Springer-Verlag GmbH, Impressum